1. Context
Cancer stem cells (CSCs) are among the most remarkable and attractive subjects in tumor biology and cancer research. CSCs are tumor-initiating cells having self-renewal and pluripotent properties and may contribute to tumor regrowth, metastasis, chemoradiotherapy resistance, and finally death (1). It has been reported that CSCs are involved in several malignancies such as leukemia and multiple solid cancer types such as colorectal cancer (CRC).
CRC is the third leading cause of cancer death worldwide, and its 5-year relative survival rate is only 8%, regardless of diagnostic and therapeutic advances (2). Colon CSCs are characterized by the expression of multiple markers such as the polycomb gene BMI1 (B cell-specific Moloney murine leukemia virus integration site 1) (3).
The BMI1 protein is a member of the polycomb group (PcG) proteins, which assembles into polycomb repressive complex 1 (PRC1) and acts as a transcriptional repressor of the Ink4a/Arf locus (4). The Ink4a/Arf locus has two distinct gene products, p16 (Ink4a) and p14 (Arf), which act as key tumor suppressors and control important physiological processes such as apoptosis and stem cell self-renewal (5).
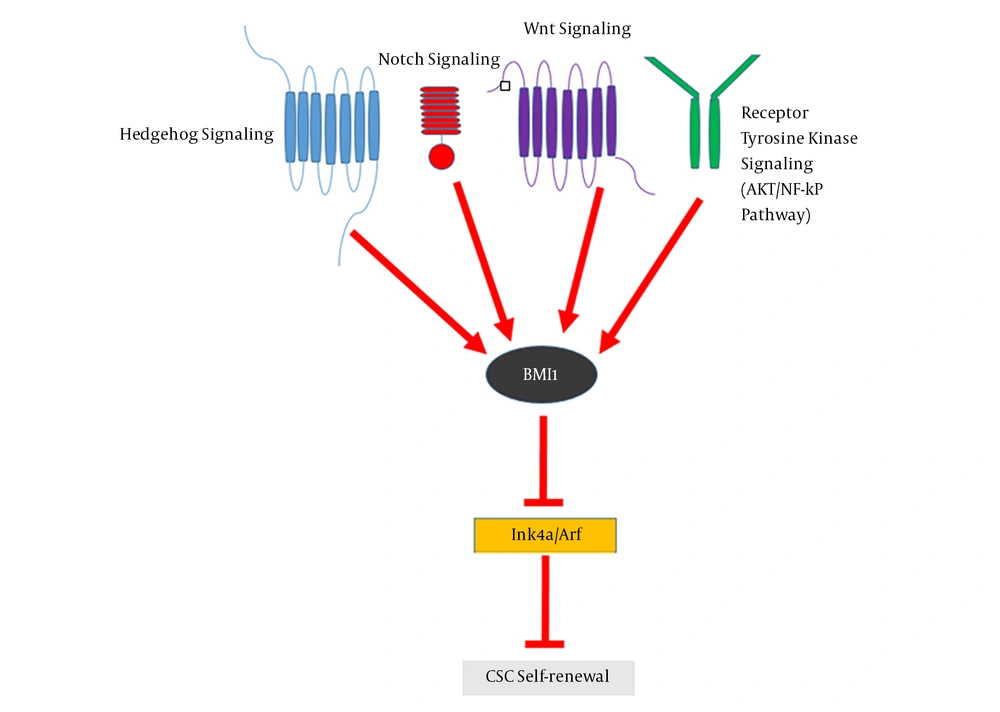
BMI1 is a key regulator of CSC self-renewal and plasticity (6) and resides downstream of several signaling pathways such as wnt/β-catenin, notch, and hedgehog, which play important roles in maintaining the growth, proliferation, and functional integrity of CSC (7). Since BMI1 is overexpressed in many cancer types, including CRC (8) and plays an essential role in stem cell self-renewal and survival; it stands out as a promising target for controlling CSC function (9). The miRNAs are small endogenous non-coding RNAs that play important roles in normal and cancer stem cells (10). The overexpression of BMI1, as a result of deregulated miRNAs, could result to cancer progression and metastasis. The present review summarized some functions of BMI1 in CSCs, especially colon CSCs, with an overview of miRNA regulation of BMI1 in different types of cancer.
2. Evidence Acquisition
The literature review of the present study utilized more than 80 articles published since 2002 in online databases, including PubMed, ScienceDirect, Medline, Google Scholar, and Scopus, using relevant keywords such as: BMI1, cancer stem cell, miRNA, and colorectal cancer. The present study investigated the relationship between BMI1 and miRNA dysregulation in various types of cancer. The role of BMI1 as a colon cancer stem cell marker is another topic, which has been explored in different related articles.
It was assumed that the association of BMI1 with miRNA could be useful as a therapeutic target and more identification of molecular mechanisms are involved in cancer.
3. Results
3.1. Genetic and Protein Structure of BMI1
BMI1 has been recognized as a target of the Moloney virus insertion and identified as an oncogene that cooperates with c-Myc in the initiation of B-lymphoid tumors (11). The BMI1 gene is localized on the short arm of chromosome 10 (10p11.23), and consists of 10 exons and 9 introns. The BMI1 protein consists of 326 amino acids with molecular weight of 36.8 kDa and contains a conserved RING finger domain in its N-terminal end, which mediates stable interaction with RING1B (E3 ligase) to stimulate its activity (12). In addition, it contains a conserved helix-turn-helix-turn (H-T-H-T) motif (13), which is essential for prompting telomerase activity and immortalization of the human epithelial cell (14) and a PEST (proline (P), glutamic acid (E), serine (S), and threonine (T)) domain at the C-terminal (9) that acts as proteolytic signals leading to protein degradation (15). The deletion of PEST-like domain, which is rich in proline-serine (PS) residues, increased BMI1 stability and pro-oncogenic actions in human mammary epithelial cells (16).
3.2. BMI1 as an Epigenetic Repressor Plays an Important Role in the Self-Renewal Mechanism of CSCs and in Epithelial to Mesenchymal Transition (EMT)-Induced Cancer Stemness
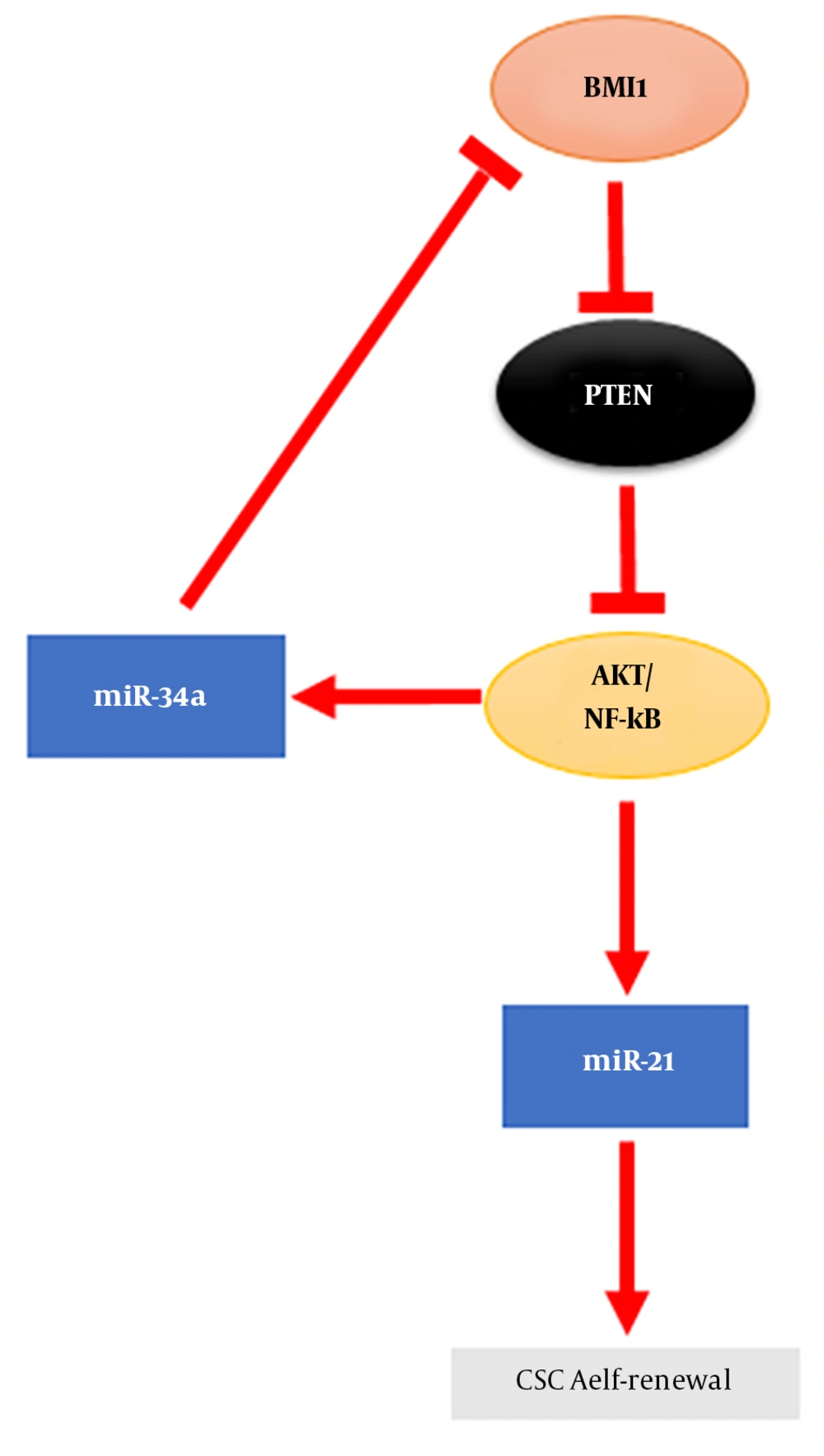
Normal stem cells have self-renewal and pluripotency properties, which are similar to CSCs as a small portion of tumor cells in tumor mass (17). CSCs are involved in tumorigenesis, metastasis, chemoradiotherapy resistance, and tumor relapse (18, 19). Increasing evidences have shown that BMI1 plays a significant role in the proliferation, self-renewal, and differentiation of several types of human stem and progenitor cells (12). It has been indicated that BMI1 is crucial for the maintenance of self-renewal, symmetrical cell division, and proliferation induction in various types of stem cells, including adult hematopoietic stem cells (HSCs) and adult peripheral and central nervous system neural stem cells (NSCs) (20). Moreover, BMI1 is a marker of distinct intestinal stem cell (ISC) populations (21), which are dormant, radio resistant, and proliferate strongly after radiation damage (22). BMI1 is not only constantly necessitated for the self-renewal of diverse tissue-specific stem cells, but is needed for the proliferation of cancer cells in the same tissues (23). The central role of BMI1 in promoting the self-renewal of various types of stem cells as well as CSCs indicates that a common mechanism regulates the self-renewal and maintenance of these cells. Self-renewal mechanisms that allow stem cells to tolerate their proliferative capacity usually include proto-oncogenic pathways such as Wnt, Hh, Notch, and the polycomb group genes (24). It has been shown that the components of these signaling pathways are mutated or overexpressed in many malignancies and could contribute to cancer cell proliferation (25). BMI1 is downstream of Wnt, Hh, and Notch signaling pathways (26). In gastric cancer, BMI1 maintains the self-renewal ability of CSCs by activating the AKT/NF-kB signaling pathway (27). BMI1 up-regulates Nanog and promotes the self-renewal capability of breast CSCs through the NF-kB pathway (28). This signifies the involvement of BMI1 in another self-renewal pathway in CSCs. BMI1 as an epigenetic repressor, can suppress p16Ink4a and p19Arf (a homolog of human p14Arf) through the induction of histone 2A (H2A) ubiquitination and histone methylation by cooperating with different PRC1 components (29). The p16Ink4a protein inhibits the binding of Cyclin D to CDK4/6, leading to the suppression of retinoblastoma (Rb) activity and induction of cell cycle arrest (30). Moreover, p19Arf can prevent cell cycle progression by activating the p53 gene (31). Nonetheless, Xu et al. showed that BMI1 functions are independent of Ink4a/Arf repression in hepatic carcinogenesis, suggesting a link between BMI1 and other oncogenic signals (32). Altogether, a crosstalk between these signaling pathways and epigenetic regulation has shown that BMI1 acts as a key factor in CSCs self-renewal, downstream of these signaling pathways (Figure 1) (2). Accumulating data show that BMI1 plays a crucial role in the EMT-induced stem cell-like property of tumor cells. The EMT mechanism is often activated during cancer development and it promotes the self-renewal ability (33). The increased expression of TWIST1 and BMI1 in association with the down-regulation of E-cadherin and p16Ink4a through chromatin remodeling is a mechanistic elucidation of the association between EMT and cancer stemness (34). Moreover, it has been revealed that the knockdown of either TWIST1 or BMI1 can reverse EMT and decrease stem-like properties (35). BMI1 contributes to the metastatic phenotypes of CSCs through expression alterations of E-cadherin and other molecules such as N-cadherin and vimentin, which result to unfavorable clinical consequences (36).
3.3. Association Between miRNA Dysregulation and BMI1 in Different Types of Cancer
It has been recognized that BMI1 is involved in the proliferation, invasion, metastasis, and chemosensitivity of cancer cells (37). Increased expression of BMI1 in numerous types of cancer has revealed that BMI1 plays significant roles in cancer initiation and development. The overexpression of BMI1 as an oncogene has been shown in leukemia (38) and various solid tumors like CRC (39). The regulation of BMI1 expression by miRNAs has been shown in a number of malignancies (Table 1). miRNAs are endogenous small non-coding RNA, which play important roles in normal and CSCs (10). Accumulating evidences indicate the role of dysregulated miRNAs as oncogenes and tumor suppressors in tumor development that suggesting their possible applications as tumor biomarkers (40-44). It has been shown that BMI1 expression repression by miRNAs leads to EMT suppression and sensitize tumor cells to chemotherapy in various types of cancer (supplementary file appendix 1). miRNAs are involved in modulating chemo-resistance and the self-renewal characteristic of CSCs in cancers. The stemness properties of gastric cancer cells is regulated by miR-21 and miR-34a (Figure 2) (27). In glioma, the decrease in self-renewal of CSCs is due to BMI1 expression inhibition by miR-128 (45). Zhang et al. demonstrated a miRNA signature related to colon CSCs stemness markers like BMI1 (46). Therefore, miRNAs are identified as prominent regulators of BMI1 expression in association with the stemness characteristics of cancer cells (47).
| miRNAs | Types of cancer | Results of BMI1 repression | References |
|---|---|---|---|
| miR15 and miR16 | Ovarian | Significant decrease in ovarian cancer cell proliferation and clonal growth | (48) |
| miR-30e* | Gastrointestinal | miR-30e* regulation of BMI1 expression mediated by tumor-associated macrophages in gastric cancer progression | (49) |
| miR-135a | Pancreatic ductal adenocarcinoma | Induced G1 arrest and apoptosis | (50) |
| miR-183 | Pancreatic ductal adenocarcinoma | Decreased expression of CDK2 and CDK4 | (51) |
| miR-320a | Nasopharyngeal carcinoma | Inhibition of cell proliferation, migration, and invasion | (52) |
| miR-429 | Renal cell carcinoma | Growth and metastasis suppression | (53, 54) |
| miR-429 | Glioblastoma | Inhibition of cell proliferation and invasion | (55) |
| miR-203 and miR-200c | Melanoma | Reduced cell invasion, tumor sphere formation, and increased expression of E cadherin | (56, 57) |
| miR-200c | Bladder | Increase expression of E-cadherin and EMT inhibition | (58) |
| miR-200c | Breast | Enhanced survival signaling | (59) |
| miR-139-5p | Bladder | Inhibiting self-renewal of bladder CSCs | (60) |
| miR-200b and miR-15b | Tongue | Reversed the phenotype of EMT in chemotherapy-resistant tongue squamous cell carcinoma cell lines | (61) |
| miR-218 | Colon | Inhibiting proliferation and inducing apoptosis | (62) |
| miR-508-3p | Colon | TGF-β induced EMT inhibition | (63) |
| miR-494-3p | Oral squamous carcinoma cells | Promotion of cellular senescence | (64) |
| miR-487b | Lung | Impaired WNT signaling | (65) |
| miR-21 | Gastric | Increased self-renewal, migration ability, and chemotherapy resistance | (27) |
3.4. BMI1 Role in CRC with Therapeutic Implications
CRC is the second most common cause of cancer in women and the third in men, responsible for considerable morbidity and mortality (66). Several studies have proven that BMI1 is up-regulated in CRC and associated with initiation, malignant development, and poor survival of CRC (67).
Although the prognostic importance of BMI1 expression in CRC is contradictory, several studies have introduced BMI1 as a prognosis marker for CRC (68). The level of BMI1 mRNA in circulation may serve as a non invasive prognostic biomarker for monitoring distant metastasis in CRC (69). In addition, Li et al. have indicated that the increased level of the BMI1 protein has a significant link with poor prognosis in colon cancer (70). Another study suggested that a combination of BMI1 and FSCN1 could be a novel prognostic indicator in CRC (71).
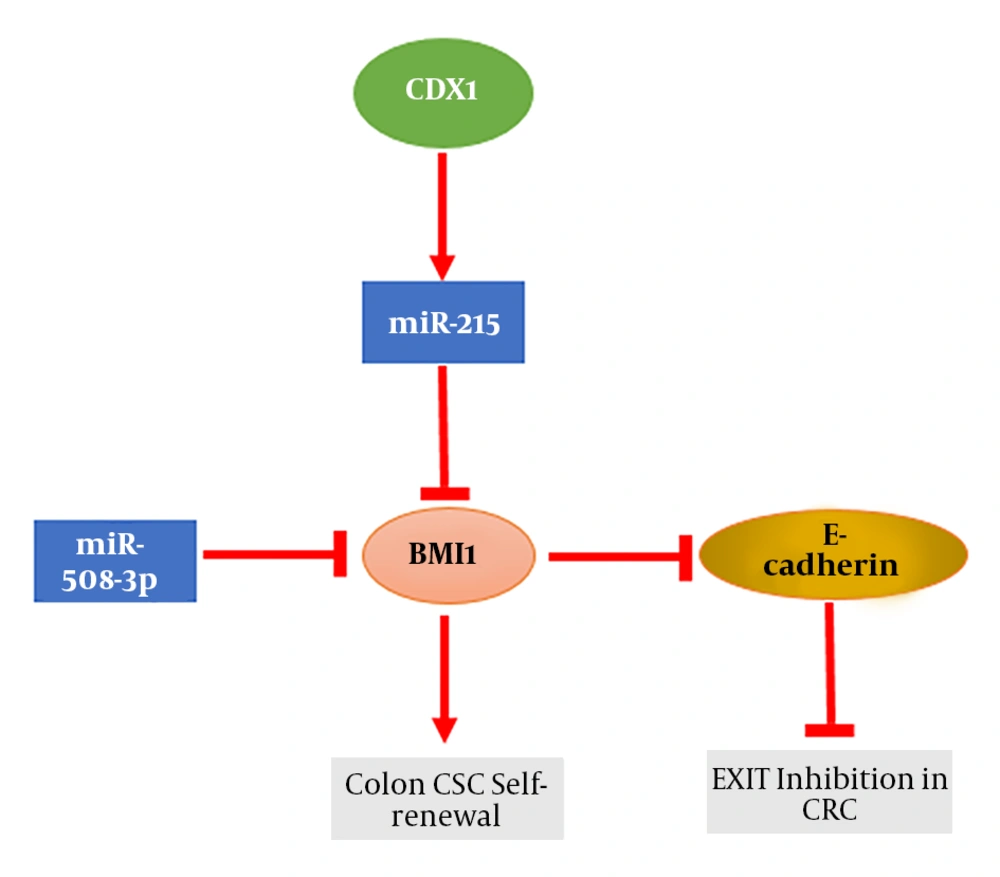
In 2007, colon CSCs isolated from bulk tumor cells were identified, using renal capsule transplantation in immunodeficient NOD/SCID mice, showing the ability to induce tumor regrowth upon serial transplantation, thereby providing strong evidence for the hierarchical model of human colon cancer (72). Network analysis showed that enrichment in zinc finger DNA binding protein and transcriptional regulator genes in BMI1 expressing tumor cells in CRC patients is consistent with stem cells self-renewal and pluripotency (71). Colorectal CSCs are plastic cells involved in intratumoral heterogeneity and are identified by chemoradiotherapy resistance, as well as metastastatic and self-renewal activity (73). BMI1 mediates histone ubiquitination and is indispensable for colon cancer cells proliferation in vitro and in vivo (74). BMI1 is overexpressed in many CSCs including colon CSCs (75). Furthermore, BMI1 is up-regulated in circulating colon cancer cells, which show stem cell like properties (76). BMI1 expression is induced by IL-1β in colon cancer and is involved in the IL-1β/Zeb1 pathway, which is critical for the self-renewal ability of colon CSC (77). BMI1 up-regulation by HES1 mediates EMT through the PTEN/Akt/GSK3β pathway in colon cancer (78). NF-kβ and β-catenin are involved in the generation of colon CSCs from tumor cells (79). It has recently been demonstrated that Fas signaling induces colon cancer stemness markers such as BMI1 (80). Therefore, BMI1 is a valuable therapeutic target for controlling the oncogenic potential of CSCs. For the first time, Kreso et al. reported that the self-renewal ability of colorectal CSCs can be targeted with PTC-209, a small-molecule inhibitor of BMI1, which leads to CRC growth and metastasis reduction without systemic toxicity (39). They revealed that by targeting BMI1, human colon CSCs in mouse xenografts can be successfully eradicated. Moreover, they showed that BMI1 knockdown by short hairpin RNA (shRNA) can diminish human CRC growth and reduce the self-renewal of colorectal CSCs. Furthermore, it has been demonstrated that targeting ZEB1, SALL4, and BMI1 via miR-508-3p can induce E- cadherin expression and inhibit cell migration, invasion, and EMT in the mesenchymal subtype of CRC (63). Using high-throughput miRNA profiling in the CRC cell line (HTC116) revealed that miR-215 directly targets CDX1 mRNA (81). CDX1 is an important mediator of differentiation in normal colon and CRC and regulates the expression of intestine and enterocyte-specific genes (82). MiR-215 acts as a target of CDX1 in colon cancer and by repression of BMI1, is involved in promoting tumor cell differentiation (Figure 3). Moreover, some drugs and negative regulators have been identified to decrease BMI1 expression (supplementary file appendix 2). By using PTC’s proprietary GEMSTM technology platform, Cao et al. demonstrated that the inhibition of BMI1 expression can selectively suppress the self-renewal of CSCs rather than normal stem cell, suggesting a safety therapeutic outcome in comparison to other BMI1 inhibitors such as histone deacetylase inhibitors (HDACi) and artemisinin (9).
4. Conclusions
CSCs are a sub-population of tumor cells that share similarity such as self-renewal and pluripotency with normal stem cells and are responsible for initiation, maintenance, and relapse of various tumors. It has been shown that CSCs become resistant to chemotherapy and radiotherapy; therefore, undesirable clinical outcomes of CSCs highlight the significance of therapeutic strategies for targeting them in various types of tumor including CRC. Increasing evidences revealed that BMI1, as an epigenetic repressor, is important in maintaining the properties of CSCs and plays a critical role in self renewal, differentiation, and tumor initiation of CSCs by silencing major tumor suppressors such as p16, p14, and PTEN. The association of miRNA dysregulation and, subsequently, BMI1 hyperactivation revealed the substantial promotion of the self renewal capacity, as well as EMT and tumor growth in various types of tumors. Improvement of clinical outcomes can be achieved by conducting more investigations on the molecular mechanisms underlying the regulation of BMI and other reliable biomarkers that are associated with pathways driving self-renewal in colon CSCs.