1. Background
Nearly 1.4 million people were suffering and 693900 died from colorectal cancer (CRC) in 2012 around the world. It is the third common cancer among men and the second one among women, but its overall prevalence is higher in men. It is more prevalent in whites. Australia/New Zealand, Europe, and North America have the highest and Africa and South Central Asia have the lowest incidence rate around the world (1). In Iran, the third leading cause of death is cancer and gastrointestinal cancers are the second prevalent ones (7/100000) (2, 3). The median survival of CRC is 30 month, but there are different prognostic factors that affect the survival rate of the patients like early detection, chemotherapy, clinical and histological factors, pathological grading, primary localization of the tumor, and number of metastatic sites (4). One of the criteria that improves the prognosis is complete resection of tumoral surgical margins (5). Resection of 1-centimeter (cm) margin is the gold standard of liver metastatic colorectal resection (6). However, there are a number of surgical factors that can alter the results and patient’s survival rate. In this study, we attempt to assess the relativity among surgical margin status and the stage of disease in Iranian patients suffering from CRC.
2. Methods
This is an observational cross-sectional uni-center study to assess the clinical and pathological characteristics of patients with colorectal cancer during 2009 to 2015 in Tehran. All the patients with pathological diagnosis of CRC based on pathologist diagnosis by non-probable judgmental sampling method were included in the study and all the patients, who received only chemotherapy or partitive care or had tumor surgery in another center, were excluded. A checklist including 88 variable about the demographic and clinical and pathological factors was filled by the pathology specialist after performing the surgery by surgeon. Based on the pathology result of the biopsy, the patients were divided into mucinous and non-mucinous adenocarcinoma groups and histologically arranged in 4 different grade; grade I: Well-differentiated, grade II: Moderately differentiated, grade III: Poorly differentiated and grade IV: Non-differentiated. The patients were staged in 10 different stages according to TNM Classification of Malignant Tumors Staging Protocol 8th edition (T stands for primary tumor site, N describes regional lymph nodes that are involved, and M is for existence of metastasis).
Surgical margin status was defined in 11 different characteristics. All data were unanimous and coded by a random coding soft wear. No extra intervention out of National Comprehensive Cancer Network (NCCN) version 1.2018 guideline was performed for the patients and all the steps of the study were performed based on national ethical standards involving human subjects and in accordance with Helsinki Declaration of 1964 and later versions revised in 2000. All the patients who participated in the study signed the informed consent form and all data were preserved by code and anonymously. The study was conducted under supervision and approval of Milad Hospital Ethical Committee.
To analyze demographic data, we used descriptive tests like frequency and mean and to declare the relativity between surgical margin status and independent variables according to the type of our variables, we used non-parametric correlation test (Spearman’s rho) by SPSS version 16. P value less than 0.05 was considered significant.
3. Results
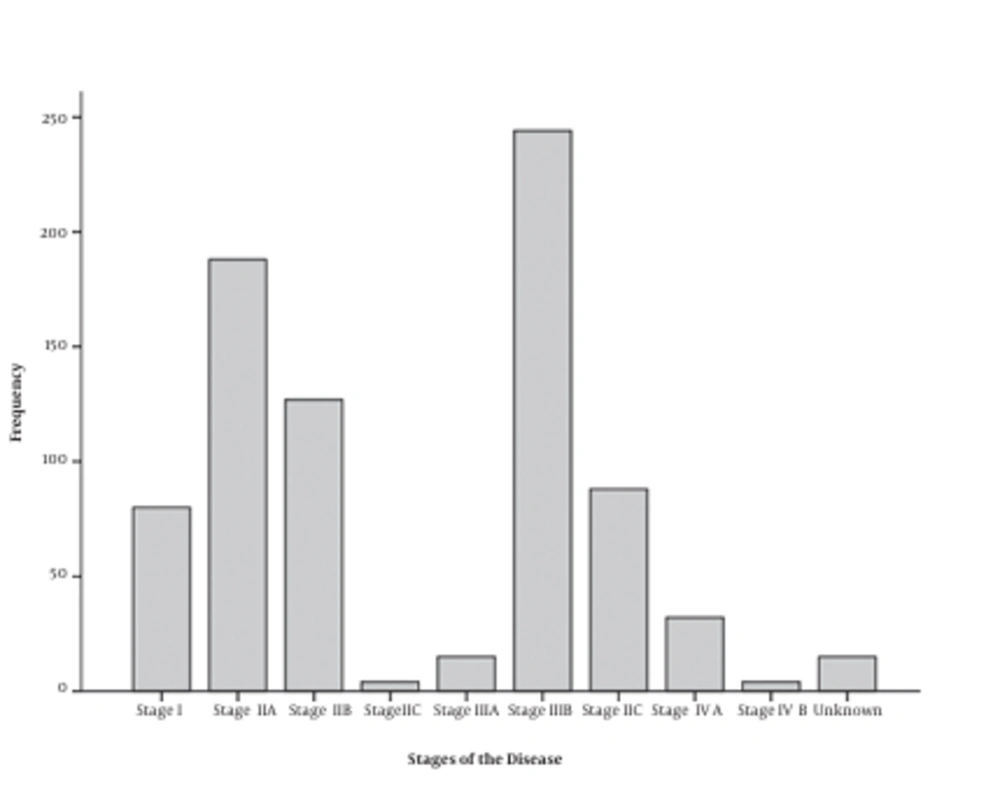
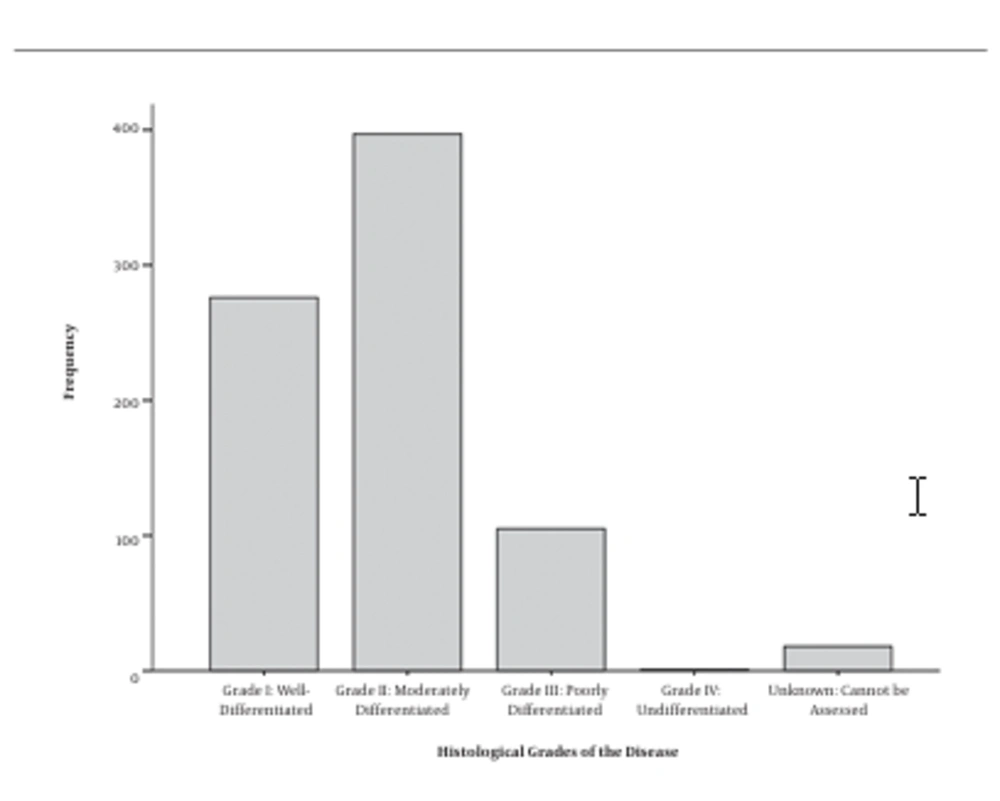
A total of 797 people with CRC participated in this study, 165 (20.7%) of whom were histologically typed as mucinous adenocarcinoma and 632 (79.3%) were diagnosed as non-mucinous adenocarcinoma. The mean age of the patients was 59.5 ± 13.8 years old (male: 59.3 ± 14.1, female: 64.1 ± 15.0). 61.7% were male and 38.3% were female. The highest prevalent diagnosing stage was IIIB (30.6%) (Figure 1) and most of them had distal and proximal free margin (54.6%) and were histological moderately differentiated (49.8%) (Figure 2). Details of the demographic data are mentioned in Table 1.
| Frequency | Percentage | |
|---|---|---|
| Surgical margin status | ||
| All surgical margin free | 143 | 37.7 |
| Proximal margin free | 5 | 1.3 |
| Distal margin free | 2 | 0.5 |
| Proximal margin free, distal margin free | 207 | 54.6 |
| Margin cannot be assessed; unknown | 20 | 5.3 |
| Proximal margin free, distal margin involved | 1 | 0.3 |
| All surgical margin free, proximal margin free: Distal margin free | 1 | 0.3 |
| Staging | ||
| Stage I | 80 | 10.0 |
| Stage IIA | 188 | 23.6 |
| Stage IIB | 127 | 15.9 |
| Stage IIC | 4 | 0.5 |
| Stage IIIA | 15 | 1.9 |
| Stage IIIB | 244 | 30.6 |
| Stage IIIC | 88 | 11.0 |
| Stage IV A | 32 | 4.0 |
| Stage IV B | 4 | 0.5 |
| Unknown | 15 | 1.9 |
| Sex | ||
| Male | 234 | 61.7 |
| Female | 145 | 38.3 |
| Valid | 379 | 100.0 |
| Missing | 418 | |
| Total | 797 | |
| Histological type | ||
| Mucinous | 165 | 20.7 |
| Non-mucinous | 632 | 79.3 |
| Total | 797 | 100.0 |
| Histologic grade | ||
| Grade I: Well-differentiated | 276 | 34.6 |
| Grade II: Moderately differentiated | 397 | 49.8 |
| Grade III: Poorly differentiated | 105 | 13.2 |
| Grade IV: Un-differentiated | 1 | 0.1 |
| Unknown: Cannot be assessed | 18 | 2.3 |
| Total | 797 | 100.0 |
Patient’s Characteristics Data Summery
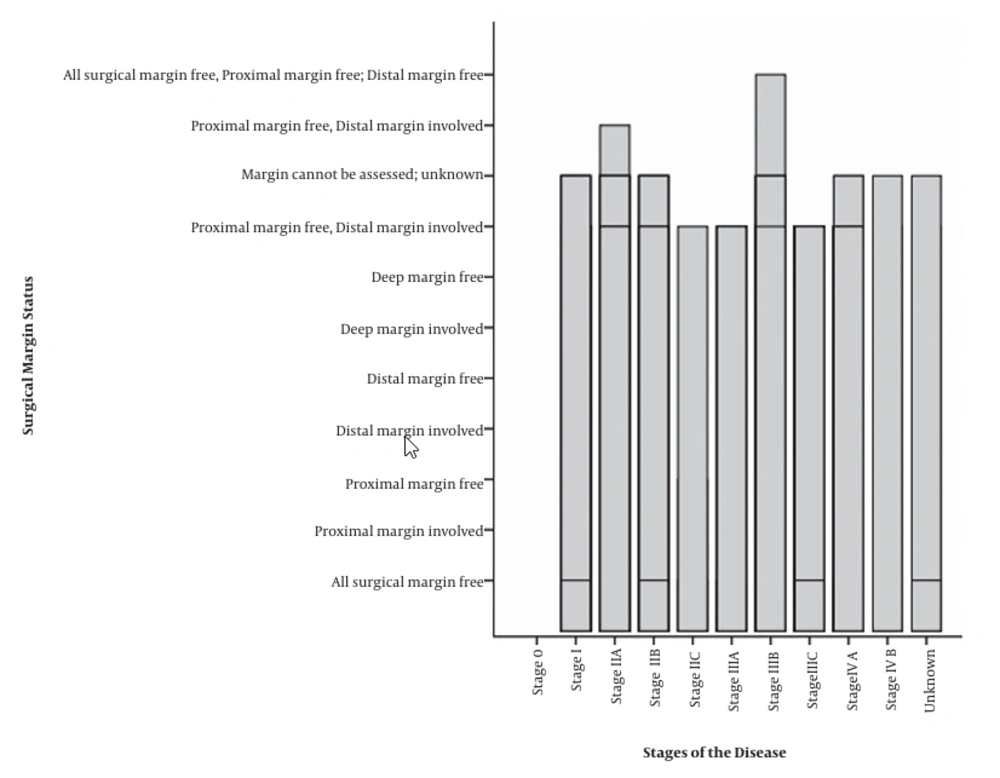
Frequencies of surgical margin status in different stages of the disease are displayed in Figure 3.
In all the age groups, stage IIIB was the most prevalent one except in 30 to 40-year-old patients that were mostly diagnosed with stage IIA disease. Stage IIIB had the highest prevalence among both female and male and also mucinous and non-mucinous patients (Table 2).
| Staging | Age Group, y | Sex | Histological Type | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 - 20 | 20 - 30 | 3 - 40 | 40 - 50 | 50 - 60 | 60 - 70 | 70 - 80 | 80 - 90 | 90 - 100 | 110 - 120 | Total | Male | Female | Total | Mucinous | Non-mucinous | Total | |
| Stage I | |||||||||||||||||
| Count | 0 | 3 | 8 | 10 | 18 | 13 | 10 | 1 | 1 | 0 | 64 | 43 | 37 | 80 | 12 | 68 | 80 |
| % of total | 0.0 | 0.4 | 1.2 | 1.5 | 2.7 | 1.9 | 1.5 | 0.1 | 0.1 | 0.0 | 9.5 | 5.4 | 4.6 | 10.0 | 1.5 | 8.5 | 10.0 |
| Stage IIA | |||||||||||||||||
| Count | 0 | 2 | 14 | 23 | 27 | 52 | 35 | 5 | 1 | 0 | 159 | 109 | 79 | 188 | 32 | 156 | 188 |
| % of total | 0.0 | 0.3 | 2.1 | 3.4 | 4.0 | 7.7 | 5.2 | 0.7 | 0.1 | 0.0 | 23.6 | 13.7 | 9.9 | 23.6 | 4.0 | 19.6 | 23.6 |
| Stage IIB | |||||||||||||||||
| Count | 0 | 3 | 6 | 18 | 36 | 22 | 20 | 5 | 0 | 0 | 110 | 84 | 43 | 127 | 26 | 101 | 127 |
| % of total | 0.0 | 0.4 | 0.9 | 2.7 | 5.3 | 3.3 | 3.0 | 0.7 | 0.0 | 0.0 | 16.3 | 10.5 | 5.4 | 15.9 | 3.3 | 12.7 | 15.9 |
| Stage IIC | |||||||||||||||||
| Count | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 4 | 2 | 2 | 4 | 1 | 3 | 4 |
| % of total | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.6 | 0.3 | 0.3 | 0.5 | 0.1 | 0.4 | 0.5 |
| Stage IIIA | |||||||||||||||||
| Count | 0 | 1 | 1 | 3 | 2 | 3 | 3 | 0 | 0 | 0 | 13 | 11 | 4 | 15 | 2 | 13 | 15 |
| % of total | 0.0 | 0.1 | 0.1 | 0.4 | 0.3 | 0.4 | 0.4 | 0.0 | 0.0 | 0.0 | 1.9 | 1.4 | 0.5 | 1.9 | 0.3 | 1.6 | 1.9 |
| Stage IIIB | |||||||||||||||||
| Count | 2 | 6 | 10 | 27 | 62 | 54 | 35 | 8 | 0 | 1 | 205 | 145 | 99 | 244 | 47 | 197 | 244 |
| % of total | 0.3 | 0.9 | 1.5 | 4.0 | 9.2 | 8.0 | 5.2 | 1.2 | 0.0 | 0.1 | 30.4 | 18.2 | 12.4 | 30.6 | 5.9 | 24.7 | 30.6 |
| Stage IIIC | |||||||||||||||||
| Count | 1 | 2 | 11 | 14 | 20 | 15 | 10 | 5 | 0 | 0 | 78 | 55 | 33 | 88 | 31 | 57 | 88 |
| % of total | 0.1 | 0.3 | 1.6 | 2.1 | 3.0 | 2.2 | 1.5 | 0.7 | 0.0 | 0.0 | 11.6 | 6.9 | 4.1 | 11.0 | 3.9 | 7.2 | 11.0 |
| Stage IV A | |||||||||||||||||
| Count | 0 | 0 | 3 | 4 | 10 | 3 | 7 | 1 | 0 | 0 | 28 | 17 | 15 | 32 | 10 | 22 | 32 |
| % of total | 0.0 | 0.0 | 0.4 | 0.6 | 1.5 | 0.4 | 1.0 | 0.1 | 0.0 | 0.0 | 4.1 | 2.1 | 1.9 | 4.0 | 1.3 | 2.8 | 4.0 |
| Stage IV B | |||||||||||||||||
| Count | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 2 | 4 | 1 | 3 | 4 |
| % of total | 0.0 | 0.0 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.3 | 0.3 | 0.5 | 0.1 | 0.4 | 0.5 |
| Unknown | |||||||||||||||||
| Count | 0 | 0 | 1 | 1 | 2 | 2 | 5 | 0 | 0 | 0 | 11 | 7 | 8 | 15 | 3 | 12 | 15 |
| % of total | 0.0 | 0.0 | 0.1 | 0.1 | 0.3 | 0.3 | 0.7 | 0.0 | 0.0 | 0.0 | 1.6 | 0.9 | 1.0 | 1.9 | 0.4 | 1.5 | 1.9 |
| Total | |||||||||||||||||
| Count | 3 | 17 | 56 | 101 | 179 | 164 | 127 | 25 | 2 | 1 | 675 | 475 | 322 | 797 | 165 | 632 | 797 |
| % of total | 0.4 | 2.5 | 8.3 | 15.0 | 26.5 | 24.3 | 18.8 | 3.7 | 0.3 | 0.1 | 100.0 | 59.6 | 40.4 | 100.0 | 20.7 | 79.3 | 100.0 |
Prevalence of Different Stage of Disease in Different Age Group and Sex and Histological Types of Disease
Mucinous adenocarcinoma histological type was 53.8% more in male than female (male: N = 100, 12.5%, female: N = 65, 8.2%). Number of patients with non-mucinous adenocarcinoma lesion was 4.03 times more than patients with mucinous adenocarcinoma lesion (N = 541, 80.1%, N = 134, 19.9%).
The stage of the disease and its histological type and grade were significantly correlated (P = 0.02, and < 0.0001, respectively). There were also significant correlation between histological grade and type of the disease (P = 0.04), but no significant correlation was observed between other variables (Table 3).
| Histologic Grade | Surgical Margin Status | Disease Stage | Histological Type | |
|---|---|---|---|---|
| Histologic grade | ||||
| Correlation coefficient | 1.000 | 0.092 | 0.203b | -0.071a |
| P value | - | 0.064 | 0.000 | 0.046 |
| N | 797 | 404 | 797 | 797 |
| Surgical margin status | ||||
| Correlation coefficient | 0.092 | 1.000 | -0.005 | -0.080 |
| P value | 0.064 | - | 0.915 | 0.110 |
| N | 404 | 404 | 404 | 404 |
| Disease stage | ||||
| Correlation coefficient | 0.203b | -0.005 | 1.000 | -0.108b |
| P value | 0.000 | 0.915 | - | 0.002 |
| N | 797 | 404 | 797 | 797 |
| Histological type | ||||
| Correlation coefficient | -0.071a | -0.080 | -0.108b | 1.000 |
| P value | 0.046 | 0.110 | 0.002 | - |
| N | 797 | 404 | 797 | 797 |
Correlation Between Surgical Margin Status and Disease Pathological Factors Among Patients with CRC
4. Discussion
In this assay, we have analyzed the relativity between the status of surgical margin in patients with colorectal cancer and their disease stage.
There are some known negative prognostic factors like positive surgical margin, margins > 5 cm, multiple metastases and their site, age > 60 years old, CEA (carcinoembryonic antigen), advance TNM stage of disease, etc. (5, 7-10).
It is not still completely clear that weather palliative or un-palliative margin resection (R1 - R2) or R0 according to Union for International Cancer Control (UICC) criteria is a more important prognostic factor or the width of surgical margins in a case that there is a tight relationship between narrow margins and extensive disease. It seems complicate to find a single surgical prognostic factor in patients with CRC (6, 9, 11-16).
In our study, the mean age of the patients and the most prevalent stage of diagnosis was higher than earlier studies either global or local studies that can be the result of lack of systematic program for gastro-intestinal (GI) and CR cancer early detection in Iran that emphasize the necessity of GI screening systems (3, 17-20).
In this study, 50 to 60 years old age group is the most populated group both in male and female that is equal to other study that was performed in 2014 in Tehran, but less than an Irish study in 2018 (18, 21).
In our survey, stage IIIB is the most populated in all age groups except 30 to 40 years old group that is higher than the Irish study. And another one in 2012 in Tehran that showed stage IV is the most prevalent stage (44.5%) (3, 21).
Surgical margin status and stage of the disease are two important prognostic factors in disease recurrence and survival; there are different ideas about effectiveness of each one and some studies claim the biology of tumor and tumor factors have higher impact on survival than surgical margin status (SMS) (11, 22). Another study found that SMS is not directly effective on survival rate, but it is the reflection of more acute disease (15). In a study, free surgical margins was an independent prognostic factor (6) and in another one, recurrent rate was not related to margin’s width (12). Some studies declare that free surgical margin is more important than the width of margins and it is related with extensive disease (13, 14, 23). But, they both are effective on survival pattern in potentially curable patients (5). On the other hand, some studies found no significant difference among SMS and SMW and overall survival, but showed that free surgical margins independently from their width are related with patient’s disease free survival (11, 24).
Historically, 1 cm margin resection was the gold standard of colorectal liver metastasis, but now there are discussions on the efficacy of 1 mm resection. In 2009, Vandeweyer et al. claimed that there is a significant difference in 5-year patient survival between patient with ≤ 1 mm and > 1 mm margin resection; although, their 5-year disease free survival was not significantly different (6). Another study found that 1 cm margin resection is the golden standard, but free surgical margin is another independent factor (12). There is also another one that say 1 mm surgical margin is optimum and there is no statistically difference among the survival rate of patients with free surgical margins and patients with > 1 mm margins (9).
In this study, the stage of the disease and its histological type and grade were significantly correlated. There were also significant correlation between histological grade and type of the disease, but there was no significant relativity among surgical margin status histological type and grade and stage of the disease.
4.1. Conclusions
Surgical margin status and stage of the disease are challenging prognostic factors in disease recurrence and survival.
The patients who participated in this study had meanly higher age and stage of diagnosis than earlier studies either global or local. It can be due to lack of systematic program for early detection of CR cancer in Iran that emphasizes the necessity of GI screening systems.