1. Background
In recent years, axillary lymph node dissection (ALND) has been largely replaced by sentinel lymph node biopsy (SLNB) as the standard technique for axillary staging in patients with early stage breast cancer. This is because many trials have shown that SLNB has comparable accuracy with ALND, while causing lower rates of morbidity (1).
In women with T1 - T2 breast cancer and clinically negative axillary nodes by ultrasonography and physical examination, SLNB is able to identify more than 90% of positive nodes, with a false-negative rate of less than 10% (2).
Nowadays, many patients with locally advanced breast cancer (especially individuals with positive axillary lymph nodes) are planned to receive neoadjuvant chemotherapy (NAC) before surgery (3).
However, there is a large debate over the role of SLNB in patients, who have received NAC. Although small institutional studies have reported different false negative rates (FNRs) for SLNB after NAC, a recent meta-analysis has shown that SLNB can be considered as an accurate and reliable tool in order to make treatment decisions for patients, who have received NAC (4).
As the majority of the published papers have included both clinically positive and negative axillary nodes (by ultrasonography and physical examination) in their studies, FNRs of the SLNB following NAC might have been underestimated (5).
Some trials, however, have exclusively enrolled clinically node positive patients in their studies, which has resulted in higher false negative rates (6-10).
In this study, accuracy and feasibility of sentinel lymph node biopsy in patients with breast cancer presented with clinically positive axillary nodes were evaluated.
2. Methods
2.1. Study Design
We conducted a cross sectional study on patients with breast cancer treated at Milad Hospital of Tehran, Iran from June 2014 to February 2015.
Clinically node positive patients (proven by biopsy), who became clinically node-negative by ultrasonography and physical examination following neoadjuvant chemotherapy and had been dissected up to 3 lymph nodes according to sentinel lymph node pattern, were included in the study.
Exclusion criteria were as follow: inflammatory breast cancer, metastatic disease, and patients with clinically positive axillary nodes after NAC. Furthermore, patients whose sentinel nodes could not be identified were excluded.
Tumor staging was done based on the TMN staging system. All patients underwent complete ALND after SLNB.
All patients signed the consent form, and the study was approved by the research Ethics Committee of Milad Hospital.
2.2. Sentinel Lymph Node Biopsy
We used Technetium-99 for axillary lymph node mapping. To do so, 0.4 mL 30 MBq (0.5 - 0.8 mCi) 99mTc tin colloid was injected in day of surgery at 3 to 4 sub-areolar and sub-dermal areas. SLNs were determined, using Gamma probe in the operating theatre.
The node showing the highest radioactivity as well as all radioactive nodes with a count ≥ 10% of the highest radioactive node were removed and sent for frozen section diagnosis.
2.3. Statistical Analysis
We used a 2 × 2 contingency table to analyze the feasibility of SLNB (sensitivity, specificity, false negative ratio, and accuracy). STATA statistical software (version 13.0, StataCorp LP, Texas, USA) was used for statistical analysis.
2.3.1. False Positive (FP)
Patients with positive SLN, but negative ALN.
2.3.2. False Negative (FN)
Patients with negative SLN, but positive ALN.
2.3.3. True Negative (TN)
Patients with negative SLN and ALN.
2.3.4. True Positive (TP)
Patients with positive SLN and ALN.
2.3.5. Sensitivity
Number of patients, who had positive SLN and ALN per number of patients who had negative SLN but positive ALN plus number of positive SLN and ALN pat ient (TP/ [FN + TP]).
2.3.6. Specificity
Number of patients with negative SLN and ALN per patient, who had positive SLN and negative ALN plus patients with negative SLN and ALN (TN/ [FP + TN]).
2.3.7. Negative Predictive Value (NPV)
Number of patients with negative SLN and ALN per patients with negative SLN and positive ALN plus patients with negative SLN and ALN (TN/ [FN + TN]).
2.3.8. Positive Predictive Value (PPV)
Number of patients with positive SLN and ALN per patient, who had positive SLN and negative ALN plus patients with positive SLN and ALN (TP/ [FP + TP]).
3. Results
Among 52 patients, who entered the study, 47 patients had been dissected up to 3 lymph nodes according to sentinel lymph node pattern. Their characteristics are shown in Table 1.
| Variables | Number | Frequency, % |
|---|---|---|
| Age | ||
| 50 - 60 | 20 | 42.6 |
| 60 - 70 | 27 | 57.4 |
| Estrogen receptors | ||
| Positive | 26 | 55.4 |
| Negative | 19 | 40.4 |
| Missing | 2 | 4.2 |
| Progesterone receptors | ||
| Positive | 21 | 44.8 |
| Negative | 24 | 51.0 |
| Missing | 2 | 4.2 |
| Her2 Status | ||
| Positive | 31 | 65.9 |
| Negative | 14 | 29.9 |
| Missing | 2 | 4.2 |
| Lymph node dissection | ||
| ALND | ||
| Positive | 16 | 34.0 |
| Negative | 31 | 66.0 |
| SLND | ||
| Positive | 21 | 44.7 |
| Negative | 26 | 55.3 |
| Pathologic complete response | ||
| Yes | 8 | 17.0 |
| No | 39 | 83.0 |
Following neoadjuvant chemotherapy, pathologic complete response (PCR) was observed in 8 patients (17.0%) (Table 2).
| Variables | Number of Subjects Clinical Staged Before NAC | Number of Subjects Pathological Staged After NAC and Surgery | P Value |
|---|---|---|---|
| T-stage | 0.0001 | ||
| T 0 | 0 | 9 (19.2) | |
| T 1 | 0 | 19 (40.4) | |
| T 2 | 0 | 15 (31.9) | |
| T 3 | 22 (46.9) | 4 (8.5) | |
| T 4 | 25 (53.1) | 0 | |
| N-stage | 0.0001 | ||
| N 0 | 0 (0) | 27 (57.4) | |
| N 1 | 23 (49.0) | 16 (34.0) | |
| N 2 | 24 (51.0) | 4 (8.5) | |
| N 3 | 0 | 0 | |
| Overall stage | 0.0001 | ||
| 0 | 0 | 8 (17.0) | |
| I | 0 | 13 (27.6) | |
| IIA | 0 | 12 (25.5) | |
| IIB | 0 | 10 (21.2) | |
| IIIA | 23 (47.9) | 4 (8.5) | |
| IIIB | 24 (52.1) | 0 | |
| IV | 0 | 0 |
Abbreviation: NAC, neoadjuvant chemotherapy.
In this study, positive sentinel lymph nodes are in 21 patients and negative sentinel lymph nodes are in 26 patients.
In all 26 patients with negative sentinel lymph node, the results of pathologic examination of axillary lymph node dissection also showed no lymph node involvement.
In 16 out of 21 patients, who had a positive result in frozen examination of sentinel lymph node, the pathological evaluation of non-sentinel nodes were also positive. In 5 patients with positive sentinel nodes, the pathological examination of non-sentinel nodes were negative and did not involve metastasis (Table 3).
| Non-Sentinel Node with Metastasis | Non-Sentinel Node Without Metastasis | Total | |
|---|---|---|---|
| Positive sentinel lymph nodes | 16 | 5 | 21 |
| Negative sentinel lymph nodes | 0 | 26 | 26 |
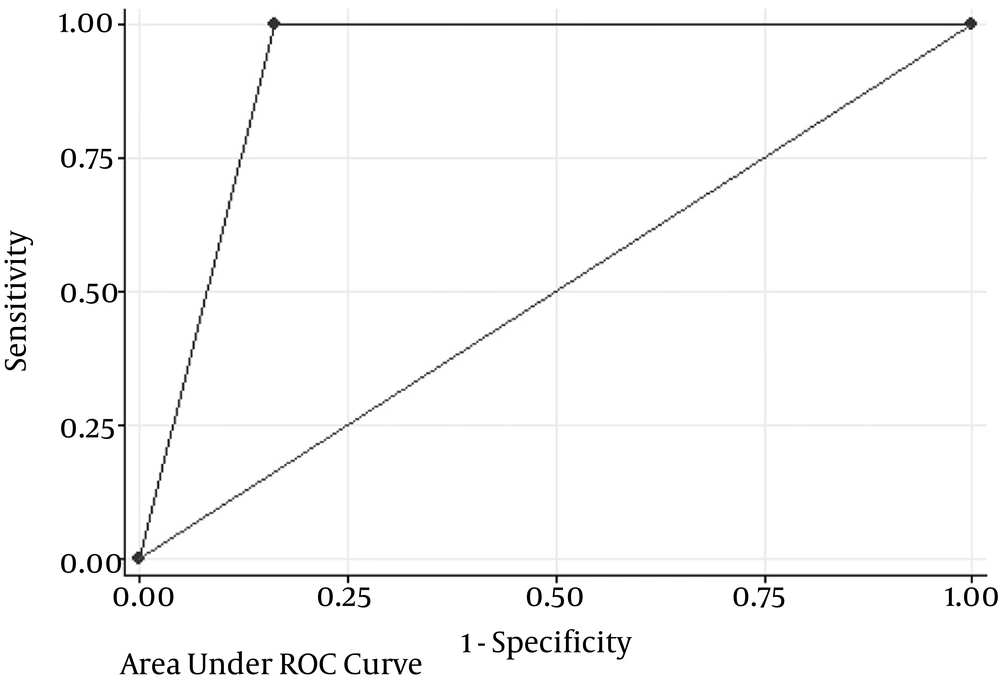
We achieved a sensitivity of 100% (16/16), false-negative rate of 0% (0/21), a negative predictive value of 100% (26/26), and an overall accuracy of 89.4% (42/47) (Table 4 and Figure 1).
| Value | 95% CI | |
|---|---|---|
| Median number of removed lymph nodes (range) | 3 (3 - 7) | |
| Sensitivity | 100% (16/16) | 79.4 - 100 |
| Specificity | 83.8% (26/31) | 66.3 - 94.5 |
| Negative predictive value | 100% (26/26) | 86.7 - 100 |
| Positive predicative value | 76.2% (16/21) | 52.8 - 91.8 |
| False positive rate | 16% (5/31) | 35.9 - 7.22 |
| False negative rate | 0 | 0 |
| Accuracy (area under ROC curve) | 89.4% | 76.9 - 97.6 |
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
4. Discussion
Feasibility and accuracy of SLNB after preoperative NAC is controversial (11). A recent meta-analysis suggested that SLNB can be used as a feasible test in node-negative patients (4). Furthermore, large clinical trials and multicenter studies in clinically node negative (by ultrasonography and physical examination) patients have reported similar false negative rates for SLNB before and after NAC (9, 10).
The value of SLNB after NAC is more of a question in clinically node-positive diseases, and some experts consider it contraindicated in these patients. This inaccuracy has several possible reasons. Neoadjuvant chemotherapy may cause lymphatic fibrosis and hence, alterations in the lymphatic drainage pattern. Furthermore, it is possible that chemotherapy affects the nodes in a non-sequential pattern, and sentinel nodes might be negative before non-sentinel nodes.
The findings of this study showed acceptable accuracy and false negative rate of SLNB, as no false negative case (negative in SLNB and positive in ALND) was observed.
Sensitivity, specificity, accuracy, and false negative rates were reported as 100%, 83.8%, 89.4%, and 0%, respectively.
According to the previous studies, false negative rates for SLNB after neoadjuvant chemotherapy in cN0 patients vary from 0% to 20%. For patients with clinically positive nodes, this figure can be as high as 30% (4, 11).
Two large clinical trials have evaluated the accuracy of SNLB after NAC in clinically node-positive patients: ACOSOG Z1071 (9) and SENTINA (10).
In the ACOSOG Z1071 trial, overall FNR was 12.6% and this rate varied based on the number of dissected lymph nodes: 31.5% when 1 SLN was dissected, 21% when 2 were dissected, and 9.1% when more than 3 nodes were dissected.
In the SENTINAL trial, FNR was reported as 24.3% for patients with one node resection and 18.5% for those with two resected nodes.
According to both studies, FNR could be less than 10% when more than 3 SLNs were resected (9, 10).
In the patients of this study, the false negative rate of 0% was achieved. The positive pathological and clinical response was higher in the present study comparing others. Previous studies have reported a positive pathological rate around 40% after neoadjuvant therapy of patients with clinically positive nodes (12). One explanation of this difference can be the limited number of cases in the current study.
4.1. Conclusions
Although previous studies have reported that SLNB after neoadjuvant chemotherapy in patients with breast cancer with positive nodes might have a higher rate of false-negative results, the present study showed that it could be feasible and accurate in this subset of patients.