1. Background
Sarcomas are relatively rare cancers of mesenchymal origin, but they are often incurable at the late metastatic stage (1). Immune response modulation by cytokine genes is one of the strategies for cancer treatment. Host immune responses are complicated because of the involvement of different cell types in immune response. Critical steps for enhancing anti-tumor responses can be induced by the administration of a single or a few cytokines (2-4). Controlling blood concentration and biological activity, which can be induced by the cytokine, are the advantages of recombinant cytokine administration. Usually, patients with cancer must receive a large amount of recombinant protein to maintain the required blood concentration of biological activity because of cytokine instability. Local gene therapy could induce local immunologic responses with minimum toxicity and strong systemic anti-cancer effects (4-6).
Interleukin-12 (IL-12) is a multiple biological effect cytokine; initially named as a cytotoxic lymphocyte maturation factor (CLMF) or natural killer cell stimulatory factor (NKSF), it is produced by stimulated macrophages (7-10). IL-12 has several biological effects on human T cells and natural killer (NK) cells, including the direct stimulation of the production of IFN-γ and other cytokines from peripheral blood T and NK cells. Induced IFN-γ by IL-12 can up-regulate the expression of MHC class I and II molecules, adhesion molecules, such as intracellular adhesion molecules(ICAM-1), and transcription factors, such as T-box expressed in T cells (T-bet) (11-16). The production of anti-angiogenic chemokines, such as IFN-γ inducible protein (IP-10, CXCL10) and monokine induced by IFN-γ (MIG, CXCL9) in endothelial cells can increase via IL-12 stimulation. The endogenous production of IFN-γ is required for the anti-tumor effect of IL-12 in most cases. On the whole, IL-12 is one of the most potent cytokines for cancer immunotherapy, and IL-12 gene therapy is, thus, expected to be effective on cancers (12, 13).
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an important hematopoietic growth factor and immune-modulator that has prominent effects on the functional activities of various circulating leukocytes. GM-CSF is produced by a mixture of cell sorts, including macrophages, T cells, endothelial cells, and fibroblasts upon receiving immune stimuli. Despite the fact that the GM-CSF production can act in a paracrine fashion to recruit circulating neutrophils, monocytes and lymphocytes can enhance their capacities in host protection. Recent investigations are centered on the administration of GM-CSF as an immune adjuvant for its ability to enhance dendritic cell (DC) maturation and function as well as macrophage activity. Initially, GM-CSF was described as an element that mediated growth, differentiation, survival and functional activities of macrophages, granulocytes, and other leukocytes. As an outcome of these effects, GM-CSF has been utilized as a part of adjuvant tumor treatments (17-19).
The aim of this study was to investigate the effect of simultaneous gene therapy with IL-12 and GM-CSF in the regression of tumor masses in a mouse model.
2. Methods
2.1. Plasmid Amplification and Isolation
A murine GM-CSF expression vector (pUMVC1-mGM-CSF) and a murine IL-12 expression vector (pUMVC3-mIL-12) were purchased from Aldevron Company (Aldevron, Fargo, ND, USA). The size of Murine GM-CSF plasmid DNA is 4423bp and contains CMV IE promoter, and the size of murine IL-12 plasmid DNA is 6247 bp and contains CMV IE promoter.
The pUMVC3-mIL-12 was amplified in an Escherichia coli DH5α strain obtained from drug applied research center (Tabriz, Iran). PUMVC1-mGM-CSF was amplified in an Escherichia coli DH5α strain, too. Plasmids were extracted according to TENS miniprep protocol. The purified plasmids were detected by gel agarose electrophoresis. DNA concentration was determined by measuring absorption at 260 nm, using a NanoDrop 1000 Spectrophotometer (Wilmington, DE, USA).
2.2. Cell Culture
WEHI-164, a BALB/c mouse fibrosarcoma cell line, was purchased from the Pasteur institute (Tehran, Iran). Skin fibroblasts from mice are the biological source of WEHI-164 cells. The cells are adherent with fibroblast morphology. The cells were cultured in RPMI-1640 medium (Sigma, Germany) supplemented with 10% fetal bovine serum (Sigma, Germany) in the presence of penicillin (100 U/mL) and streptomycin (100 µg/mL, Sigma, Germany), and incubated in a humidified incubator with 5% CO2 at 37°C.
2.3. In Vitro Transfection Studies and Determination of Transfection Efficiency
The WEHI-164 cells were seeded into a 6-well plate at a density of 4 × 105 cells/well. After 48 hours, cells were washed with phosphate-buffer solution (PBS) and 2 mL RPMI-1640 medium (containing no FBS and antibiotics) was added to wells. Then, 6 µg of the mIL-12 gene was diluted in 250 λ OptiMem in a microtube and 10 λ Lipofectamine 2000 was diluted in 250 λ OptiMem in a separate microtube. After 5 minutes, microtubes were gently mixed together and incubated at room temperature for 20 minutes. Then, the cells were incubated at 37°C for 6 hours. After 6 hours, the complexes were aspirated and replaced with culture medium. Antibiotic selection was applied after 48 hours (G418) (Thermo) for transfected cell isolation. Stable selection was completed after 7 to 10 days. The mIL-12 levels in the cell culture medium were detected by mouse IL-12 ELISA kit according to the manufacturer’s instructions (Koma Biotech company, Korea).
The amount of the protein was determined as picogram per mL. The same procedure was carried out for the m-GM-CSF gene and GM-CSF expression by mouse GM-CSF ELISA kit according to the manufacturer’s instructions (Koma Biotech Company, Korea).
2.4. Animal Experiments
Female Balb/c mice (6 - 8 weeks old) were purchased from Pasteur Institute (Tehran, Iran).
In this study, the mice were randomly divided into 4 groups and each group consisted of 7 animals (Table 1).
| Groups | Treatment |
|---|---|
| Control group | The Balb/c mice received 106 wild tumor cells. |
| GM-CSF group | The Balb/c mice received 106 tumor cells expressing GM-CSF. |
| IL-12 group | The Balb/c mice received 106 tumor cells expressing IL-12. |
| IL-12 + GM-CSF group | The Balb/c mice received 0.5 × 106 tumor cells expressing IL-12 and 106 tumor cells expressing GM-CSF simultaneously. |
To study the synergic effect of gene therapy with IL-12 and GM-CSF, 0.5 × 106 tumor cells expressing GM-CSF and 0.5 × 106 tumor cells expressing IL-12 were injected subcutaneously into the right flank of the Balb/c mice to establish a tumor in the group inoculated with tumor cells expressing IL-12 and GM-CSF (IL-12 + GM-CSF group). In the control group, 106 of wild WEHI-164 cells were injected subcutaneously into the right flank of the Balb/c mice to establish fibrosarcomas. To compare the synergic effect of IL-12 and GM-CSF with each cytokine monotherapy, 106 of tumor cells expressing GM-CSF were injected subcutaneously into the right flank of the Balb/c mice in the group inoculated with tumor cells expressing GM-CSF alone (GM-CSF group). In addition, 106 tumor cells expressing IL-12 cells were injected subcutaneously into the right flank of the Balb/c mice in the group inoculated with tumor cells expressing IL-12 alone (IL-12 group). The viability of the cells used for inoculation was over 95% as determined by Trypan blue dye exclusion test. Tumor growth was monitored 3 times a week with caliper after tumor challenge. Tumor volume (mm3) was calculated by the following formula: 1/2 × (Length × width2).
2.5. RNA Extraction and Real Time PCR
Following tumor mass extraction, total RNA was extracted, using AccuZol reagent (Bioneer, Korea) as described by the manufacturer protocol. Complementary DNA (cDNA) was synthesized from 1 µg of total RNA by using oligo-dT18 primer and MMLV reverse transcriptase (Promega, USA). q-RT-PCR was performed with SYBR Premix Ex Taq (Takara Bio, Otsu, Shiga, Japan) in the Rotor-Gene 6000 system (Corbett Life Science, Australia). CDNAs were diluted 1:4 in nuclease-free water and 5 µL of diluted cDNA was added to 20 µL of PCR mixture containing SYBR Premix Ex Taq (Takara Bio, Otsu, Shiga, Japan) and 0.2 µmol/L of each specific primer (MouseIL-12p40, Mouse GM-CSF, Mouse IFN-γ, and GAPDH). GAPDH was used as a reference gene. The primer sequences are indicated in Table 2. The initial denaturation step at 95°C for 10 minutes was followed by 45 cycles at 95°C for 20 seconds and 60°C for 1 minute. Relative gene expression was calculated with the 2-(∆∆CT), using GAPDH as the endogenous expression standard.
| Primers | Sequence |
|---|---|
| MouseIL-12p40 | Forward: 5’-GAGCACTCCCCATTCCTACT- 3’ |
| Reverse: 5’-GCATTGGACTTCGGTAGATG- 3’ | |
| Mouse GM-CSF | Forward: 5’-ACCACCTATGCGGATTTCAT- 3’ |
| Reverse: 5’-TCATTACGCAGGCACAAAAG-3’ | |
| Mouse IFN-γ | Forward: 5’-TCAGCAACAGCAAGGCGAAAA AG-3’ |
| Reverse: 5’-ACCCCGAATCAGCAGCGAC TC-3’ | |
| GAPDH | Forward: 5’- CCTCGTCCCGTAGACAAAA-3’ |
| Reverse: 5’-AATCTCCACTTTGCCACTG-3’ |
2.6. Western Blot Analysis
Fifty micrograms of total protein from each sample were heated for 5 minutes at 95°C before being loaded and separated on 12% SDS polyacrylamide gels, using a mini-gel apparatus (Bio-Rad Laboratories). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore; Billerica, MA). The membranes were blocked with 5% nonfat dry milk in TBS/Tween-20 (0.05%, v/v) for 1 hour at room temperature and, then, incubated overnight at 4°C with the appropriate primary antibodies, including anti-mouse IL-12 (Biolegend, UK, London), anti-mouse β-Actin (Abcam, Cambridge, MA, UK), anti-mouse GM-CSF(Biolegend, UK, London), and anti-mouse IFN-γ (Biolegend, UK, London) in TBS/T buffer (in a 1:1,000 dilution), respectively. The procedure was followed by incubation with the appropriate HRP-conjugated secondary antibody (1:1,000 dilution; Abcam) for 2 hours at room temperature. After washing, protein bands were visualized, using enhanced chemiluminescence detection kit (GE Healthcare) and autoradiography films (Fuji Photo Film Co., Ltd., Tokyo, Japan) according to the manufacturer’s instruction.
2.7. Immunohistochemistry Analysis
Ki-67 expression level, as a proliferation index in the tumor mass, was determined by immunohistochemistry. Briefly, four-micrometer frozen sections were cut, air-dried, fixed with Acetone, and rehydrated in PBS containing 0.05% Tween-20. Nonspecific binding sites were blocked by blocking solution for 30 minutes at room temperature. Slides were incubated with primary antibody (purified anti-mouse Ki-67) (Biolegend, UK, London) for 60 minutes. Then, slides were washed in PBS/Tween-20 and incubated with HRP labeled secondary antibody (Rabbit Polyclonal secondary antibody) to Rat IgG (HRP-conjugated) (Abcam) for 30 minutes. H2O2 was added to the DAB solution (substrate solution). The slides were incubated with DAB /H2O2 for 5 minutes and washed with PBS/Tween-20. The slides were viewed with an invert microscope.
To study Ki-67 expression, image processing software was used to count both the total number of cell nuclei in each image and the number of positively stained nuclei. Data were expressed as percent of positively stained nuclei, derived from these counts. One-way ANOVA was used for statistical analysis.
2.8. Statistical Analysis
One-way ANOVA was used to determine the significant differences between groups. All statistical analyses were performed by GraphPad Prism software version 6. P value < 0.05 was considered to be statistically significant.
This study was approved by ethics committee of Tabriz University of medical Sciences.
3. Results
3.1. Confirmation of Murine GM-CSF and Murine IL-12 Expression by Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay tests were carried out for confirmation of IL-12 and GM-CSF expression by tumor cells transfected with IL-12 and GM-CSF genes. The IL-12 and GM-CSF protein concentrations in the supernatant of cell culture were assessed by spectrophotometry in 540 nm λ. The IL-12 concentration in cell culture supernatant was found to be 1000 pg/mL, and the concentration of GM-CSF in cell culture supernatant was 800 pg/mL.
3.2. Anti-Tumor Effects of IL-12 and GM-CSF in Vivo
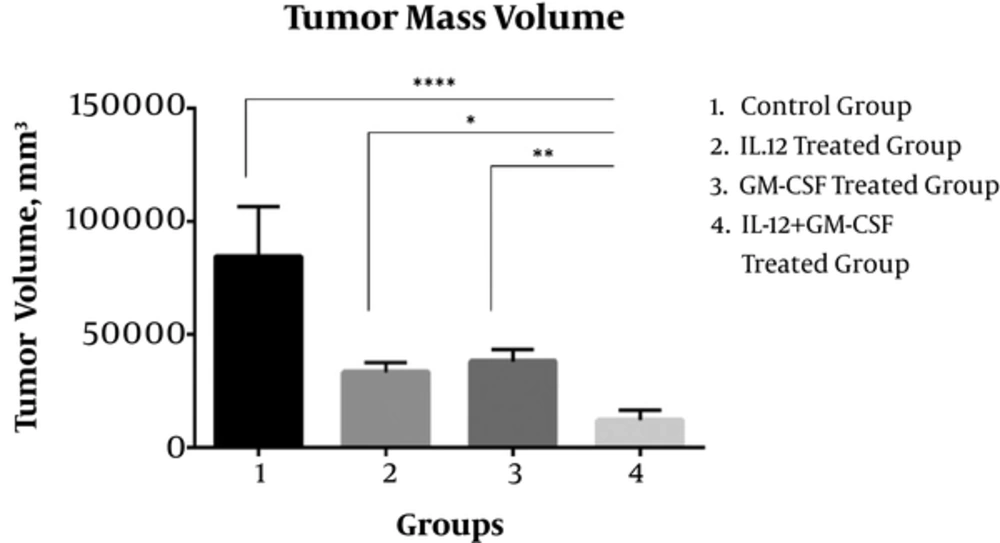
Twenty-one days after tumor inoculation, the volume of tumor masses in the IL-12 + GM-CSf group was reduced significantly in comparison to the control group (P < 0.0001) and IL-12 group (P < 0.05) or GM-CSF group alone (P < 0.01).
The mean volume of tumor masses in the control group was 84604 ± 8290 mm3 and the mean volume of tumor masses in the IL-12 + GM-CSF group was 12210 ± 1693 mm3. Tumor volume in the IL12 + GM-CSF group was reduced significantly compared to either monotherapy. Hence, gene therapy with IL-12 and GM-CSF had a significant effect on the regression of tumor masses (Figure 1). The reproducible results were obtained in repeated tests.
Synergistic Anti-Tumor Effects Induced by Combined IL-12 and GM-CSF Gene Therapy; The results showed that the tumor mass volume was significantly reduced in IL-12 + GM-CSF group compared with the control group. (P < 0.0001). Statistical significance was set at *, P < 0.05; **, P < 0.001; ***, P < 0.001, P < 0.0001**** by One Way ANOVA.
3.3. Expression of Murine GM-CSF, Murine IL-12 and Murine IFN-γ mRNA, and Protein in Tumor Tissue
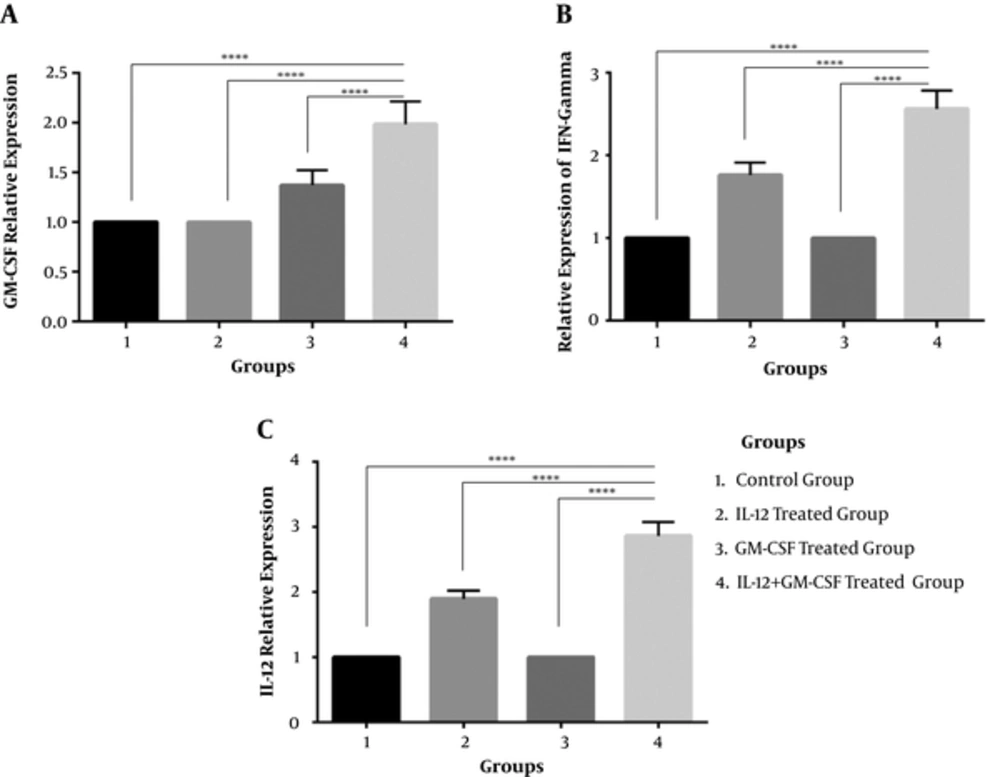
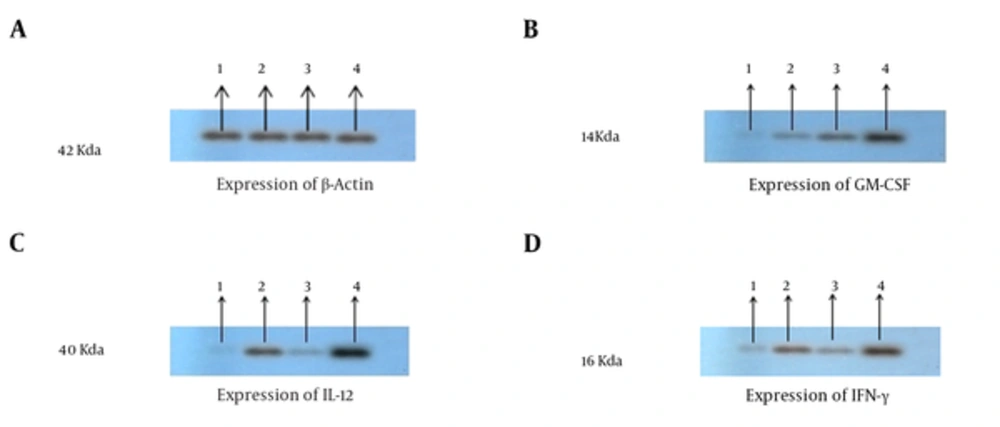
To investigate whether tumor cells were transfected with GM-CSF and IL-12, gene in the group of mice were inoculated with tumor cells expressing IL-12 and GM-CSF in vivo, and tumor masses were harvested from mice 21 days after tumor inoculation. Total RNA was extracted from the tumor masses and cell lysate was prepared to confirm IL-12, GM-CSF, and IFN-γ by real time PCR. The results of real time-PCR indicated that the expression of GM-CSF,IFN-y and IL-12 was significantly enhanced in the IL-12 + GM-CSF group in comparison to other 3 groups (with relative expression of 1.98, 2.56, and 2.86), P < 000.1, (Figure 2A, 2B, 2C). Furthermore, the results of immunoblotting showed that the expression of IL-12, GM-CSF, and IFN-γ was enhanced in the IL-12 + GM-CSF group in comparison to control group (Figure 3).
Relative Expression of GM-CSF, IFN and IL-12 Genes Was Analyzed by Real Time PCR Method. A, Relative expression of GM-CSF gene. GM-CSF gene expression is up-regulated in IL-12 + GM-CSF group (compared to the three other groups) by a mean factor of 1.98 (P < 0.0001). B, Relative expression of IFN-γ gene. IFN-γ gene expression is up-regulated in the IL-12 + GM-CSF group (compared to other 3 groups) by a mean factor of 2.56 (P < 0.0001). C, Relative expression of IL-12. IL-12 gene expression is up-regulated in the IL-12 + GM-CSF group (compared to other 3 groups) by a mean factor of 2.86 (P < 0.0001).
The Cytokine Expression Results of Western Blot: IL-12, GM-CSF, and INF-γ Expression Has Been Proved by Western Blotting Analysis; A, Proteins were equalized by use of β-actin expression; B, Western blotting results showed that GM-CSF expression was enhanced in the IL-12 + GM-CSF group compared with other groups; C, Western blotting results showed that the expression of IL-12 was enhanced in in the IL-12 + GM-CSF group compared with other groups. D, Western blotting results showed that the expression of IFN-γ was enhanced in the IL-12 + GM-CSF group compared with other groups.
3.4. Expression of Ki67 in Tumor Sections
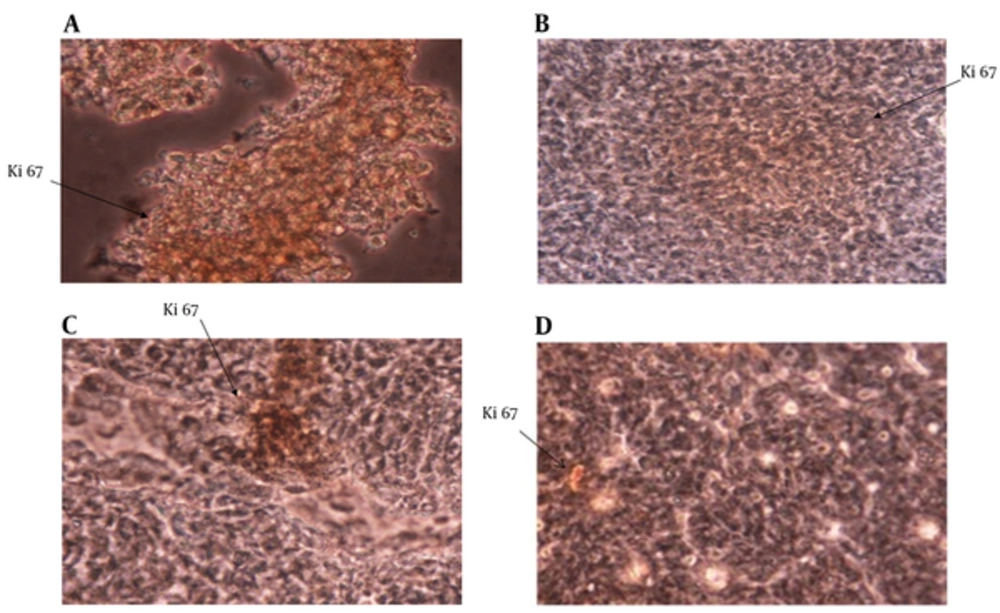
The expression of Ki67 in tumor masses was studied with immunohistochemistry staining. The results showed that Ki67 expression was significantly reduced in the IL-12 + GM-CSF group in comparison to other 3 groups (P < 0.05) (Figure 4).
Expression of Ki67 in Tumor Tissue; Ki67 expression in tumor tissue was analyzed by immunohistochemistry method. A, Expression of Ki67 in control group; B, Expression of Ki67 in IL-12 group; C, Expression of Ki67 in GM-CSF group; D, Expression of Ki67 in IL-12 + GM-CSF group Expression of Ki67 was reduced in IL-12 + GM-CSF group compared with other 3 groups.
4. Discussion
Local cytokine gene delivery was exhibited to be an effective approach for the cancer treatment (20, 21). The bystander effect of cytokine gene therapy plays a critical role in cancer therapy due to the fact that it is impossible to deliver the gene into all tumor cells. It was reported that the host’s immune system plays a critical role in the bystander effect in vivo (22, 23). So, the improvement of the host’s immune response could increase the bystander effect of the cytokine gene therapy. Helms reported that IL-12 could improve recruitment of CIK (cytokine-induced killer) cells and the increased anti-tumor activity could be achieved by combining IL-12 cytokine therapy with CIK-based adoptive immunotherapy (24). The local expression of IL-12, to maintain low serum concentrations as well as reduce systemic toxicity, could be rapidly gained by gene therapy vectors (25-28). IL-12 local concentration could not only reduce toxicity, but would be important for the establishment of anti-tumor immune responses (29).
IL-12 induces an effective cellular immune response against syngeneic tumors, which is concomitant with a local enhancement in inflammatory Th1 cytokines in the tumor microenvironment. These altered immune responses are followed by a systemic protective immunity; thus, IL-12 has proven to be a promising immunotherapeutic approach for cancer therapy (30). The anti-tumor effect of IL-12 gene therapy was dependent largely on NK cells and partially on T cells. Another appealing observation was that the effect of IL-12 on anti neo-vascularization of the tumor was also dependent on the 2 cellular components. In some recent reports, NK cells and T cells have been shown to inhibit tumor angiogenesis by production of IFN-γ (31-34). IFN-γ is a potent activator of NK cells and boosts their cytolytic activity. GM-CSF has the main capacity to stimulate proliferation, maturation, and function of anti-gen representation cells. Through local release of IFN-γ, tumoricidal macrophages and eosinophils may be recruited and activated (35, 36). In 2013, Miguel et al. showed the synergy between the GM-CSF and IL-12 in a preventive and therapeutic tumor vaccine model, using genetically modified tumor cells and the results of their study indicated the preventive vaccination gained total efficacy in terms of tumor growth inhibition and animal survival (37). Choi et al. studied the anti-tumor effect of adenovirus expressing IL-12 and GM-CSF and the results showed that an Adeno virus co-expressing IL-12 and GM-CSF induces more effective anti-tumor responses and increases survival by inducing more cytotoxic activity of T cells and production of IFN-γ compared with an Adeno virus expressing IL-12 or GM-CSF alone (38). In another study, Chang, Chen et al. demonstrated that combination therapy with IL-12 and GM-CSF could synergistically repress the tumor growth in orthotropic liver tumors by inducing various types of effectors and high expression of IFN-γ (15). The present study evaluated the therapeutic efficiency of combined cytokine gene therapy mediated by IL-12 and GM-CSF in a murine model. The results showed significant tumor mass volume regression in the IL-12 + GM-CSF group in comparison with the control group, IL-12 group, and GM-CSF group alone due to the synergic effect of IL-12 and GM-CSF, although the IL-12 and GM-CSF genes alone had a significant effect on tumor regression. Combined gene therapy caused the high level of IFN-γ due to the synergic effect of IL-12 and GM-CSF. These findings are in agreement with previously reported results and all results verify each other. IL-12 induced the Th1 responses against tumors and GM-CSF stimulated APC maturation. Results also demonstrated that gene therapy with GM-CSF and IL-12 could enhance the IFN-γ expression in the tumor site and decrease Ki67 expression in the fibrosarcoma tumor masses. The results of real time PCR and western blotting showed that the expression of IL-12, IFN-γ and GM-CSF was significantly enhanced in the IL-12 + GM-CSF group. Furthermore, gene therapy with IL-12 and GM-CSF could decrease Ki-67 expression in the fibrosarcoma tumor masses, and this finding showed that IL-12 and GM-CSF gene therapy could decrease cancerous cell proliferation. Ki-67 is a protein associated with cell proliferation and it is present in all other cell cycle phases except G0. The expression of IFN-γ induced by IL-12 and GM-CSF has a co-delivery function, because IFN-γ induction is a major factor responsible for the IL-12–induced anti-tumor effects as well as being a major cause for the IL-12–induced toxicity. One of the side effects of IL-12 is the hematological toxicity, whereas GM-CSF is a hematopoietic growth factor. Thus, there exist additional advantages for the combined IL-12 and GM-CSF gene therapy (14, 16). Additionally, a significant level of intra-tumoral IFN-γ was also observed as an inducer of 2 important anti-tumor chemokines, IP-10 and MIG (31). MIG expression in the tumor microenvironment induces T cells and NK cells to exert their anti-tumor cytotoxic activities (31).
In conclusion, combination therapy with IL-12 and GM-CSF demonstrated a promising approach for treating fibrosarcoma. Thus, combination therapy may be considered a potential therapeutic choice in the treatment of fibrosarcoma.