1. Background
Multiple myeloma (MM) is a malignant plasma cells disorder associated with accumulation of abnormal plasma cell in the bone marrow. Bone pain, anemia, renal failure, and recurrent infections are known as symptoms of MM progression, which are due to the consequences of incomplete immunoglobulin secretion (1). The exact etiology is not known, but several mechanisms have been recognized to play role in etiology of MM; factors, such as genetic instability, environmental factors, oxidative stress, and inflammation have been found to contribute to the MM disease (2, 3).
Reactive oxygen species (ROS) have a crucial role in the impairment of cellular biomolecules. DNA, lipids, and proteins of living organisms are proposed to be damaged by free radicals; a process which is mediated by single unpaired electron (4). These free radicals are produced in normal cell function and metabolisms, but when this imbalance occurs between free radicals or oxidative stress and antioxidant towards the excessive of oxidative stress, the cellular damage occurs (5). The biological cell damage, which is caused by these species, is termed oxidative stress. Cellular macromolecules damage in cancer has been shown in various in vivo and in vitro studies. Harmful effects of oxidative stress occur when the overproduction of reactive oxygen species is not eradicated by antioxidant defense in body (6).
Reactive nitrogen species (RNS) act together with ROS and augment the oxidative macromolecule damage (7). ROS/RNS cause alterations in DNA structure, such as base pair mutations, rearrangements, deletions, insertions, and sequence implication, all being accompanied by the impairment of cell function and signal transduction (8). These alterations may be related to second wild-type allele inactivation and contributed with a mutated proto-oncogene or tumor-suppressor gene that can occur during tumor promotion and progression. Also, When the DNA damage repair enzyme systems do not work properly, genome instability and mutations can occur into the cell genome (9). DNA damage is known to be the most important consequence of oxidative stress (10). Protein expression can be regulated through the redox modification of transcription when this modification is associated with DNA damage repair enzyme systems result in the increased DNA damage and higher incidence of cancer (11-13). Genomic modification in tumor cells of MM via the formation of mutations have been identified and characterized by secretion of cytokines. The studies have revealed the presence of oxidative stress in multiple myeloma (14). Measurement of 8-hydroxy-deoxyguanosine (8-OHdG) has been known as sensitive and reliable biomarker for oxidative DNA damage. Recently, most of the studies have analyzed the levels of the 8-OHdG in biological samples of patients with cancer in relation to the oxidative stress. 8-OHdG levels in patients with various cancers were also significantly higher than those in control group (15, 16).
The aim of this study was to assess changes in 8-OHdG, nitrite, and nitrate levels in patients with stage I multiple myeloma cancer and healthy individuals. Likewise, no study reported the evaluation of serum 8-OHdG levels in patients with stage I MM. In addition, we measured the 8-OHdG, as a biomarker for oxidative DNA damage in stage I multiple myeloma, in comparison to healthy group for better assessment of antioxidant/oxidative stress status in the advanced stage of multiple myeloma and treatment of newly diagnosed multiple myeloma.
2. Methods
2.1. Patients
We designed this case-control study on 34 stage I multiple myeloma patients with newly diagnosed (mean age: 69 ± 5, 12 females/ 22 males) and 35 healthy controls (mean age: 71 ± 7.0, 13 females/ 22 males) at Urmia oncology educational and research hospital, Urmia, Iran. The study procedures and protocols were approved by ethics committee of Urmia Medical University (faculty of medicine, department of clinical biochemistry, Urmia, Iran). Written informed consents were obtained from all participants prior to the study. Patients with stage I MM were diagnosed by pathology reports and analyzed serum protein electrophoresis and immunotyping (Capillarys®, Sebia, France). The international staging systems was used for staging MM patients according to serum β2-microglobulin values (β2-microglobulin less than 3.5 mg/L were considered having multiple myeloma); (Chemiluminescence, Diasorin, Liaison, Italy; detection limit: 0.12 mg/L and CV = 3.9%) (17). The Immunophenotyping distribution consisted of 22 patients with IgG kappa, 5 patients with IgG lambda, 2 patients with IgA kappa, 2 patients with IgA lambda, and 3 patients with IgM kappa. Patients who had a history of anti-oxidant or vitamins consumption, chemotherapy, heart disease, kidney disease, diabetes, chronic infections, smoking habit, and nitrate releasing drugs were excluded from study. Venous blood samples were obtained from overnight fasting. The serum was separated via centrifuge at 4000 rpm (Hermle Z360 K, Japan), transferred to the Cryo Tubes and kept -80°C until analysis.
2.2. Determination of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) Levels
Serum levels of 8-OHdG were measured by competitive ELISA kit (Cayman chemicals, Ann Arbor, Michigan, USA). This kit is a fast and sensitive competitive immunoassay to detect and quantify 8-hydroxy-deoxyguanosine in serum samples. 8-OHdG levels were evaluated according to the kit construction, using the mouse anti-mouse IgG-coated plate provided in the kit and a standard curve of 8-OHdG. The values were reported as pg/mL, the inter-assay coefficients of variation (CV) were 4.5% and detection limit of approximately was 30 pg/mL.
2.3. Determination of Nitrite (NO2-) and Nitrate (NO3-) Levels
Serum levels of nitrite and nitrate were measured in each sample according to the kit protocol as the measurement of NO biosynthesis and NO syntheses activity. Nitrate was measured as nitrite after enzymatic conversion by nitrate reducates (Cayman, Ann Arbor, MI, USA). Griess reaction was, then, used for nitrite determination. The intra-assay and inter-assay coefficients of variation (CV) were 3.4% and 2.7% with detection limit of 2.5 µmol.
2.4. Determination of Other Blood Parameters
Serum levels of creatinine, calcium, and total protein levels were directly measured with commercially available kits with standard laboratory methods (Biosystem, Barcelona, Spain).
2.5. Statistical Analysis
All results are expressed as means ± SD. Statistical analyses were analyzed, using SPSS software (version 18, SPSS Inc. Chicago, Illinois, USA). Student’s t test and one way analysis of variance were performed for analyzing and comparing the results between the groups. Pearson correlation coefficients were used for relationship between measured parameters. A P value < 0.05 was considered statistically significant.
3. Results
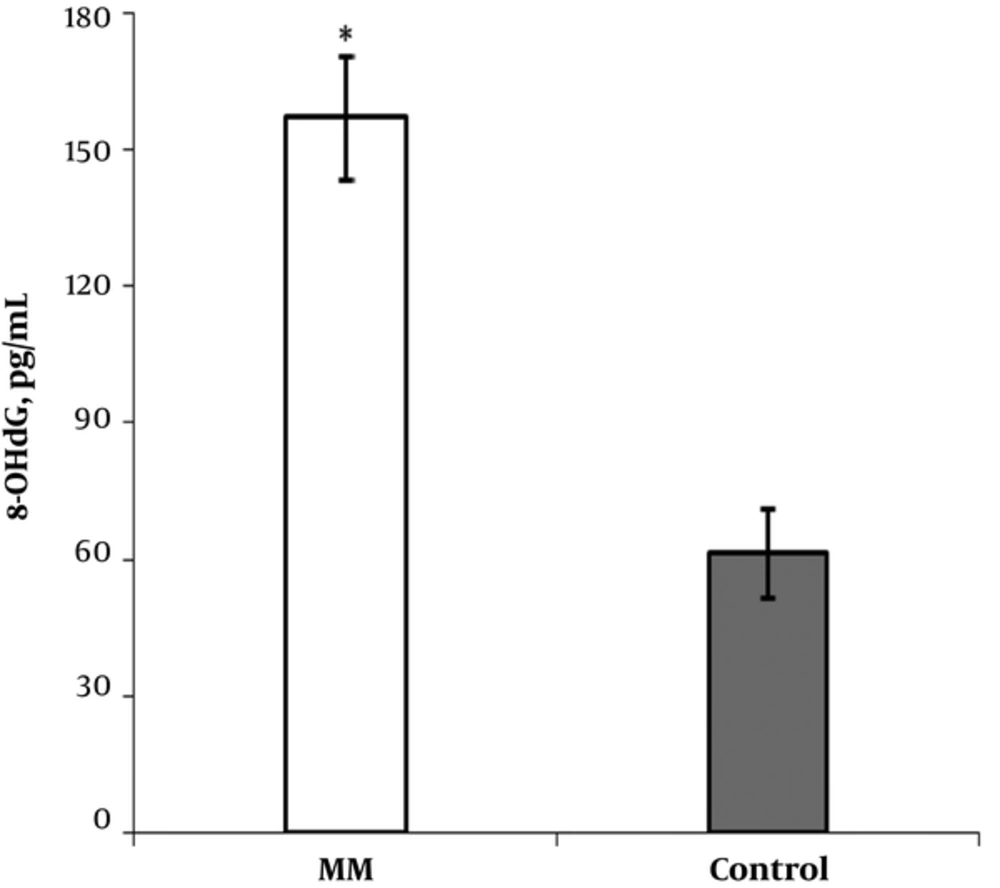
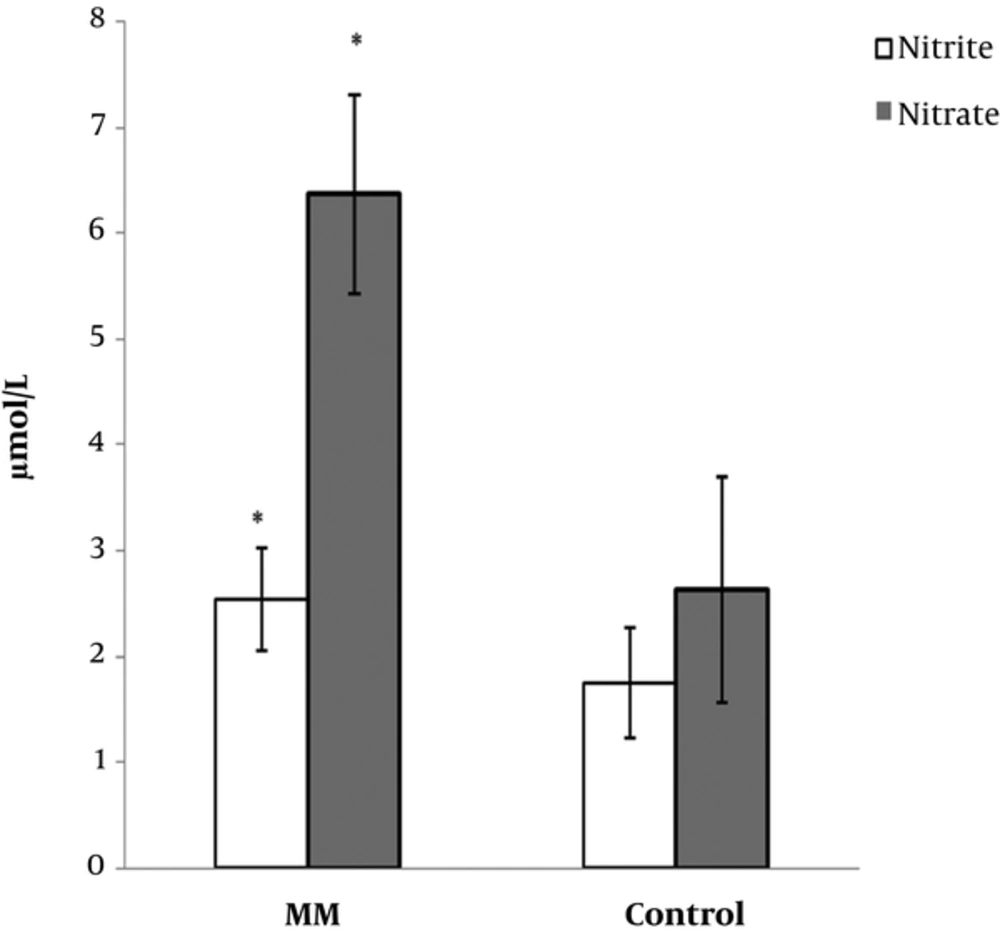
The clinical characteristics and laboratory data from patients with MM cancer and healthy subjects are shown in Table 1. The mean levels of creatinine in cancer group were higher than those of control group (P < 0.033). Similarity, serum total protein level increased in patient group compared with the control group (P < 0.001). No significant differences were determined in comparison with β2-microglobulin (P < 0.88) and calcium levels (P < 0.26) between cancer group and controls. The results of the present study revealed a significant increase of 8-OHdG levels in patients with MM cancer (157.08 ± 13.61 pg/mL) compared with controls (61.48 ± 9.93 pg/mL) (P < 0.001) (Figure 1). Similarly, we found a significant increase of nitrite and nitrate levels in patient group (2.54 ± 0.49 vs. 1.75 ± 0.52 µmol/L, P < 0.001 and 6.37 ± 0.94 vs. 2.63 ± 1.06 µmol/L, P < 0.001, respectively) compared to controls (Table 1, Figure 2). No significant correlation was identified among 8-OHdG, nitrite, and nitrate levels in patients and control group.
| Parameter | Patients | Controls | P |
|---|---|---|---|
| Age (years) | 69 ± 5.0 | 71 ± 7.0 | 0.42 |
| Male/female | 22/12 | 22/13 | 0.87 |
| Haemoglobin (g/dL) | 12.75 ± 0.77 (12.48 - 13.03) | 13.94 ± 0.79 (13.66 - 14.21) | 0.005b |
| Calcium (mg/dL) | 9.03 ± 0.33 (8.91 - 9.14) | 8.95 ± 0.22 (8.87 - 9.03) | 0.26 |
| Creatinine (mg/dL) | 0.81 ± 0.15 (0.75 - 0.86) | 0.91 ± 0.22 (0.83 - 0.99) | 0.033c |
| Serum NO2- ( µmol/L) | 2.54 ± 0.49 (2.36 - 2.71) | 1.75 ± 0.52 (1.57 - 1.93) | 0.000d |
| Serum NO3- (µmol/L) | 6.37 ± 0.94 (6.04 - 6.70) | 2.63 ± 1.06 (2.26 - 2.99) | 0.000d |
| β2-microglobulin (mg/L) | 1.70 ± 0.53 (1.52 - 1.89) | 1.69 ± 0.48 (1.52 - 1.85) | 0.88 |
| Total protein (g/dL) | 10.34 ± 0.91 (10.02 - 10.66) | 7.11 ± 0.42 (6.97 - 7.26) | 0.000d |
Comparison of Clinical Characteristics and Laboratory Data Parameters in Control Subjects and MM Patientsa
4. Discussion
Reactive oxygen species (ROS) are known as producible mediators during the normal cellular metabolism activity. Excessive production of reactive species or inadequate and impairment of antioxidant defense system can cause cellular oxidative damage (18). The oxidative damage of cellular biomolecules, such as DNA, proteins, and lipids are known to be involved in disease progression. The studies have revealed that inflammatory pathway, which are related with most chronic diseases including cancer, diabetes, and inflammatory diseases, could be triggered by chronic oxidative stress (19). The activation of some inflammatory pathways, which are associated with proliferation, chemoresistance, radioresistance, invasion, and angiogenesis of tumor cell, have been complicated with oxidative stress (20). Oxidative stress causes the activation of some regulatory genes, which are related with the expression of different genes, including inflammatory molecules and cell cycle regulatory molecules (21).
Reactive metabolites are contributed with strand breaks and cross-linking in DNA. Also, chronic oxidative stress leads to proto-oncogene and tumor-suppressor genes mutations, and then, promoting neoplastic transformation. Previous studies showed that the most commonly mutated genes, including KRAS, NRAS, and TP53 have been identified in MM patients (22). Oxidative stress is related with somatic mutations of the variety of transcription factors, including RAS, NF-κB, and p53. The mutation of these genes have been observed in some cancers and associated with proliferation of malignant cells and induced the production of pro-inflammatory cytokines, such as IL-1, and IL-6, which are involved with the progression of cancer (23). We showed the significant increase in serum 8-hydroxy-deoxyguanosine (8-OHdG) values as an oxidative DNA damage biomarker, while serum nitrite and nitrate were found to be reduced in patients with cancer compared to control group. This is the first report on the serum 8-OHdG, nitrite, and nitrate levels in patients with stage I MM. An 8-OHdG provides a useful indicator and reliable approach for the assessment of oxidative DNA damage in vivo. Elevated oxidative DNA damage in patients with MM cancer may be associated with previous studies, which have indicated the decrease in enzymatic and non-enzymatic antioxidant system as well as increase in free radicals or reactive metabolites in MM patients (24-27). The imbalance between oxidative stress/antioxidants was referred as main reason for the elevation and excessive generation of DNA damage products in MM patients. The results of the present study are in line with earlier reports of elevated DNA damage in patients with various cancers. Khadem-Ansari et al. found an increase in 8-OHdG levels in the urine of esophageal squamous cell carcinoma (28). Similarly, Diakowska et al. showed that 8-OHdG were higher in human oesophageal cancer (29). Crohns et al, likewise, reported the elevated levels of 8-OHdG in the urine of patients with lung cancer (30). A study conducted by Wei et al. showed the higher levels in serum 8-OHdG values in patients with colorectal cancer than in healthy controls (31). Cobanoglu et al. reported an increase in the levels of MDA and 8-OHdG in patients with lung cancer (32). Ito et al. investigated the urinary concentration of the 8-OHdG with clinical factors in patients with lung cancer. They demonstrated that there were higher concentrations in 8-OHdG levels in smoker patients compared with non-smoker patients. Furthermore, they indicated the lower levels of 8-OHdG in patients with stages II-IV disease compared with that of patients with stage I lung cancer (33).
Nitrite and nitrate are both eventual fates of nitric oxide (NO) oxidation, known as end-products of endogenous NO metabolism. These metabolites are produced as normal metabolites in human biological fluids. The increase in an excessive production of nitrite and nitrate in body has been implicated with decrease in anti-oxidant capacities and disease progression by the induction of cytotoxic and mutagenic effects. Studies have shown the induction of DNA damage by nitrite and nitrate when they are present in excessive concentration (34). In this study, we found increased serum nitrite and nitrate levels in MM cancer group compared with the control group. Increased oxidative DNA damage, which was determined in this study, seemed to be related with serum levels of nitrite and nitrate. Elevated reactive oxygen species may be linked as a main reason for increased serum nitrite and nitrate, which in turn could ameliorate the oxidative DNA damage in MM patients. The levels of Nitrite and nitrate were reported in several studies to be either decreased or increased in cancer. Similar to the results of the present study, Bakan et al. have reported a higher serum nitrite and nitrate levels in patients with gastric cancer compared with the healthy controls. They indicated higher increase in plasma levels of nitrate in the higher stages of cancer (35). In another study, Beevi et al. reported the significantly elevated levels of the total nitrate and nitrite in patients with oral cavity cancer compared to healthy controls (36). In contrast, Gönenç et al. found a lower serum nitrite and nitrate levels in patients with gastrointestinal cancer (37).
In the present study, we estimated the serum 8-OHdG as oxidative DNA damage. Elevated levels of 8-OHdG with increase in serum nitrate and nitrite levels may be related with reduction in antioxidant defense system. The results of the present study are in accordance with previous studies, which mentioned the impairment of the anti-oxidant enzymes in MM disease (38). This reduction may be associated with silencing several genes related with the expression of antioxidant genes. On the other hand, these findings may be correlated with mutations, which occur in DNA strand and may be involved with insufficiency of anti-oxidant enzyme activity. There was no correlation among 8-OHdG levels, nitrite, and nitrate with clinical factor in stage I MM. This elevation in serum 8-OHdG could be associated with increased ROS/RNS in MM, which is considered in the pathogenesis of MM by accumulation of DNA damage adduct. Serum nitrite and nitrate have been shown to be higher during inflammation and oxidative stress-associated disease. Studies have declared the reduction in antioxidant capacity in patients with MM. This reduction in activity of antioxidants and involvement of oxidative stress in MM cancer are in line with the present study (39, 40).
In summary, we have shown that high concentrations of nitrite and nitrate were detected in stage I MM. This increase in serum end-products of endogenous nitric oxide (NO) metabolism may severe oxidative DNA damage in MM patient. Elevated serum levels of 8-OHdG, as reliable biomarker of oxidative DNA damage in stage I of MM, highlight the possibility and relevant role of increased ROS/RNS in MM disease. These findings indicated the impairment of anti-oxidant status and may be associated with MM cancer. The measurement of 8-OHdG may provide clinically useful marker for the management of oxidative stress mediated cancer progression. However, more studies are suggested to explore the possible association between the oxidative DNA damage and antioxidant status and also describe the resulting of RNS in MM.