1. Introduction
Gross hematuria has a wide differential diagnosis (1-4). Primary prostate sarcoma, which derives from the mesenchymal cells of the prostate stroma and is responsible for less than 0.1% of primary prostate cancers, was first reported in 2003. Primitive neuroectodermal tumor (PNET) or extra-skeletal Ewing’s sarcoma is a very rare type of prostate sarcoma that mostly occurs in young adults, and is associated with a poor prognosis (5-7). PNET is classified to central and peripheral types according to their locations (3). Cluster designation (CD) 99 positivity distinguishes PNET from other entities such as rhabdomyosarcoma, lymphoma, and neuroendocrine cancer (8).
2. Case Presentation
A 37-year-old male was admitted to emergency department with intermittent painless gross hematuria from 1 month prior to admission. The medical and familial histories were unremarkable. He had no documentation of previous malignancies. Digital rectal examination demonstrated a very large prostate with poor mobility. There were no palpable lymph nodes. Ultrasonographic evaluations revealed normal kidney structure, bilateral hydronephrosis, distended Bladder filled with clot, and a huge prostate. According to orthostatic blood pressure changes and hemoglobin of 7mg/dL, conservative therapy initiated and patient was transferred to operation room (OR) urgently. Cystoscopy and bladder washing was done and bleeding origin was detected from prostate lodge.
The day after that, the patient was reevaluated in OR because of the persistent bleeding. Trans-urethral resection of prostate (TURP) (3 bites for tissue sampling), fulguration, bilateral ureteroscopy, and rectoscopy were performed and, finally, TRUS Bx of prostate was done. The patient’s serum prostate-specific antigen (PSA) level was 1.07 ng/mL, and all of the other serum tumor markers (such as carbohydrate antigen 19 - 9, carcino-embryonic antigen, alpha-fetoprotein, and lactate dehydrogenase) were normal.
Histological findings were consistent with severely inflamed prostatic urethra, papillary heperplasia, and reactive atypia with no evidence of malignancy. The PSA and immunohistochemistry (IHC) results of alpha-methylacyl-CoA racemase (AMACR) were negative and leukocyte common antigen (LCA) markers were positive in suspicious cells.
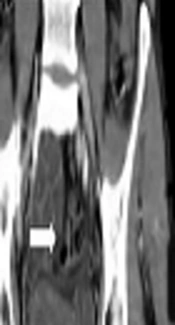
After this report and persistency of gross hematuria, computed tomography (CT) angiography for rule out of arterio-venous malformations, abdominopelvic computed tomography scan (ACTS), and magnetic resonance imaging (MRI) with intravenous contrast were done. All of them showed an 86 × 57 × 78 mm mass replacing the prostate gland, which appeared multi-lobulated nodular mass with central septation (Figure 1).
The patient subsequently underwent radical prostatectomy. Histopathology of the specimens showed prostatic tissue involved by a malignant neoplasm of small round and oval cells with scant cytoplasm suggestive of PNET (Figure 2). IHC study results on CD99 were strongly positive. Also vimentin, B-cell lymphoma 2 (BCL2), and Ki67 were positive, and CD 3, 10, 20 and 34, cytokeratins (CK) 7 and 20, CD117, high molecular weight cytokeratin (HMWCK), PanCK, progesterone receptor (PR), desmin, smooth muscle actin (SMA), S100, terminal deoxynucleotide transferase (TDT), epithelial membrane antigen (EMA), neuron specific enolase (NSE), and synaptophysin were negative in the sample.
According to definitive diagnosis and urethral involvement by tumor adjuvant chemotherapy with ifosfamide (2 mg/m2/week) started for the patient. CT was rechecked, displaying no evidence of distant metastasis till 9 months after surgery. In the 10th month, the patient referred with the symptoms of abdominal pain and severs constipation, in the abdominopelvic CT scan of a pelvic. A 32 × 75 × 112 mm mass in the lodge of prostate was seen. The patient was again subjected to 10 sessions of chemotherapy with the previous regiment, but adequate response was not obtained. The patient did not accept redo surgery and died 16 months after the initial operation.
3. Discussion
Diagnosis of PNET in the prostate is most challenging. In all prostate PNET case reports, a large-sized primary tumor is seen replacing the prostate lodge. The subjects often described the same sign and symptom as the patients with benign prostatic enlargement (9), whereas our patient presented with gross hematuria. Thus, prostate PNET should be suspected when young males refer with the complaints similar to those of benign prostatic hyperplasia, change of bowel habit or gross hematuria. The ACTS and MRI findings consist of the evidence of a heterogeneously enhanced mass in all cases. And subsequent biopsy with IHC staining is required to confirm the diagnosis (10, 11). However, the acceptable management for this entity is still debatable. The currently recommended treatment is based on chemotherapy followed by radical prostatectomy/cystoprostatectomy, and radiotherapy. However, data on the long-term follow-up have been limited; most patients were disease-free no more than 24 months after treatment (6).
Including present case, there have been only less than 10 case reports to date of prostate PNET. We report a rare, uncommon case of prostate PNET presented by intermittent painless gross hematuria. As the prognosis is very poor, medical staff should pay enough attention to the differential diagnosis, choosing the best treatment, and subjects close follow up.