1. Background
The patients with head and neck (H and N) cancers include about 5% of cancer cases diagnosed, worldwide. Also, surgery and radiotherapy are curative treatments for only about 3.2% of new cancer cases (1). These cancers lead to about 350,000 deaths, annually (2, 3). Radiotherapy is a common medical procedure used in such patients (4). Mucositis is the most common complication of radiotherapy in these patients that is defined as inflammatory and/or ulcerative injuries of oral cavity and/or stomach and intestines (5). About 83% of patients need radiotherapy and at least 60% of them get mucositis (5). According to World Health Organization (WHO), in patients with H and N cancer that received high doses of radiation (6000 - 7000 GY), grade 3 and 4 mucositis reaches to 85%, but all patients get some degrees of mucositis (6). It was reported in a study that the prevalence of severe mucositis was 35% to 65% and these patients are at risk of discontinuing the treatment (7).
Ulcerative mucositis of oral cavity is a debilitating and painful condition that causes severe pain, increasing the risk of local and systemic infection, oral and pharyngeal dysfunction and oral bleeding that effects patients’ eating, sleeping, speaking and generally, quality of life that can lead to hospitalization or increase the time of hospitalization, and enhance the medical expenses (7, 8).
As yet, some treatments proposed the management of radiation-induced mucositis; they were successful in some cases; but, there is no completely effective way to prevent mucositis during and after radiotherapy and it can be said that there is no proved prophylaxis for it (7). Therefore, most of treatments contain supportive cares like pain relief, nutritional support, and improvement of salivary status (9); proposing new treatment approaches are recommended.
Traditional systems of medicine are usually considered potential sources to find new treatments based on old knowledge (10). Persian Medicine (PM) is one of these traditional systems of medicine. Due to the 10,000-year-old background of Persian medicine (11), searching in the manuscripts of this medical doctrine, which are used for centuries, is a reasonable method for proposing and designing drugs; that is why using the experiences of traditional medicine increases the possibility of finding effective drugs up to 40%, while this is only 1% in random research studies (12, 13). WHO allowed to research on medicinal plants with long history of usage on human being with specific instructions (14).
2. Objectives
According to the etiology of mucositis and by searching the therapeutic effects of medicinal plants in different Persian references and modern medicine, a list of herbs was provided and considering criteria, lack of serious complications in recent studies, availability, affordability, accurate identification of herb and appropriate taste to patients appeal, Alcea digitata Alef. (Khatmi in Persian language) and Malva sylvestris L. (Panirak in Persian language) were chosen for this study after multi-step screening.
In Persian medicine, A.digitata was used to heal the coughs (specially for coughs due to irrigation and inflammation of pharyngeal mucosa) to decrease the swelling of mucus membranes of stomach and intestines, inflations of brain, ears and eyelids, to heal the wounds, and to relief the pain of swellings and wens (15, 16). Approved indications and usages of this herb by commission E are cough and bronchitis, inflammation of gastric mucosa, oral, and pharyngeal irritation (17).
M.sylvestris has compounds with anti-inflammatory and antioxidant effects (18). This herb is a rich source of vitamins A, B, and C and is effective in reducing the complications of common cold, especially coughs. it is also useful in treatment of inflammations of respiratory, urinary and digestive tracks, and acnes. Studies show that this herb has antibacterial, antifungal, and antiviral effects against human pathogens (16, 19). It was shown that the aqueous extract of M.sylvestris is more effective than cimetidine in treatment of gastric ulcers (18, 20). Also M.sylvestris has been used as anti-cough and diuretic orally, and as abstergent in treatment of wounds topically in Persian medicine (15). Current studies show that these plants are immune stimulants and, therefore, can be considered potential remedy for mucositis (16).
On the other hand, according to contents of PDR of Herbal Medicine, these 2 medicinal plants have been approved by Commission E and no report was found stating serious side effects (17).
According to both traditional and current evidences, the present study was designed to evaluate the efficacy of the combination of these medicinal plants on prevention of radiation-induced mucositis in the patients with H and N cancers.
3. Methods
In order to prepare compound drug, in advance, the flowers of A.digitata and M.sylvestris were purchased from an herbal shop in Tehran. They were identified and kept in the herbarium center of school of pharmacy, Tehran University of Medical Sciences with voucher numbers PMP-508 and PMP-509, respectively. Then, both plants were cleaned, powdered, and mixed; then, they were kept in 4 g sachets, containing equal portions. Also, the placebo was prepared from Avicel in form of 4 g sachets, too.
In this triple-blind parallel two-armed randomized clinical trial, 23 patients with H and N cancers, who came for radiotherapy to Imam Hossein hospital oncology clinic, were involved. They recoursed to the clinic from February 2015 to September 2016. The protocol of this investigation was approved by the ethics committee of Shahed University (Code: 41/215586) and registered in the Iranian registry of clinical trials (IRCT2014120520208N1).
Inclusion criteria consisted of age between 17 to 65 years, life expectancy over 1 year, good performance status according to Eastern cooperative oncology group (ECOG) criterion, and a minimum of 4 areas in radiation field. Patients were excluded if they had used alcohol, drugs affecting salivary gland secretions, such as anti-depressants, opioids, anti-hypertensives, anti-histamines, diuretics, mouthwashes, artificial saliva, and cigars, had had the history of chemotherapy and radiotherapy in oropharyngeal region in past 6 months, had had the history of connective tissue diseases, such as Sjogren’s syndrome, Rheumatoid arthritis and Lupus, liver or kidney diseases, major depression, diseases involving salivary glands such as diabetes, diseases causing dehydration, such as chronic diarrhea, immunosuppressive disorders, Myelosuppression, and diseases causing Aphthous ulcers. Patients were excluded too, if they had had mucositis grade 3 or 4, Candidiasis, oral Herpes, failure to treat, need to TPN or hospitalization before getting 50 GY of radiation or did not want to continue the treatment.
Eligible subjects were randomly allocated to 2 groups; those receiving the herbal compound (experimental group) or those receiving placebo (control group) 3 times per day for 7 weeks from beginning of radiotherapy to 2 weeks later.
The herbal compound and the placebo were given to patients by secretary of clinic, and patients, investigators, and statistical analyzer did not know the allocation method during the study until the data analysis.
The protocol of RCT was explained to the patients, and before participating in the study, they studied and signed the written informed consent. Patients were examined at the beginning of the study and followed up each week (2 weeks after completing the radiotherapy). There were both interview and physical examinations of the patients for gathering the required data during their visits. In the first visit, all protocols of the study and prescription of each medication were explained to the patients who met the inclusion criteria. The efficacy of treatment on mouth pain score (MPS) was assessed by using visual analog scale (VAS) and mucositis grade was evaluated by investigator according to WHO scale in every visit. The VAS was scored from 0 to 10, where 0 and 10 denoted the absence of mouth pain and severe mouth pain, respectively.
The collected data compared between 2 groups and more accurate analysis was done, using mixed statistical model with STATA software and the mean MPS; the mucositis of patients of 2 groups were compared in every week. In this integrated analysis, drug effect, time effect and time-drug interaction were surveyed and anywhere the interaction was significant, drug effect in any time, and vice versa, time effect in experimental and control group were analyzed. The p-Value under 0.05 was considered significant.
4. Results
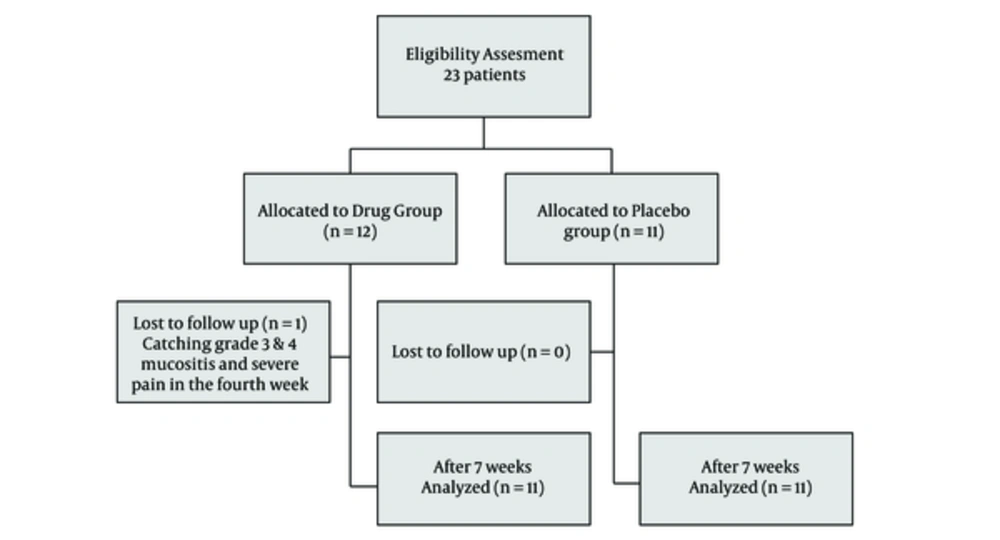
A total of 23 patients (13 males and 10 females) were enrolled in the present study and randomly allocated to 2 groups (12 in drug group and 11 in control (placebo) group). The mean age was 54.16 in experimental group and 60.45 in control group. The experimental group received the herbal compound and the control group received placebo. Only 1 patient in control group was excluded because of catching grade 3 and 4 mucositis and severe pain in the fourth week (Figure 1).
The complications were not statistically significant in 2 groups (P > 0.05). There were no significant differences between 2 drug and placebo groups in the subjects of age and age distribution as well as other demographic variables (Table 1).
| Study Group | N (Number of Included Patients) | Mean of Age ± SD | Mean of Patients Length ± SD | Mean of BMI ± SD | Dental Fillings | Non-Smokers | Hemoglobin, g/dL | Platelets, × 103/μL | WBC, × 109/L |
|---|---|---|---|---|---|---|---|---|---|
| Drug | 12 | 55.16 ± 16.73 | 1.68 ± 0.08 | 24.29 ± 5.72 | 6 | 8 | 12.88 ± 1.48 | 260.00 ± 63.43 | 5.90 ± 2.26 |
| Placebo | 11 | 60.45 ± 19.25 | 1.60 ± 0.10 | 25.46 ± 4.96 | 5 | 9 | 12.34 ± 1.37 | 303.28 ± 105.91 | 5.84 ± 0.91 |
| Whole patients | 23 | 57.69 ± 17.76 | 1.64 ± 0.10 | 24.85 ± 5.28 | 11 | 17 | 12.63 ± 1.40 | 280.20 ± 85.55 | 5.87 ± 1.71 |
| -Value | 0.4887 | 0.048 | 0.6084 | 0.565 | 0.601 | 0.4760 | 0.3469 | 0.9483 |
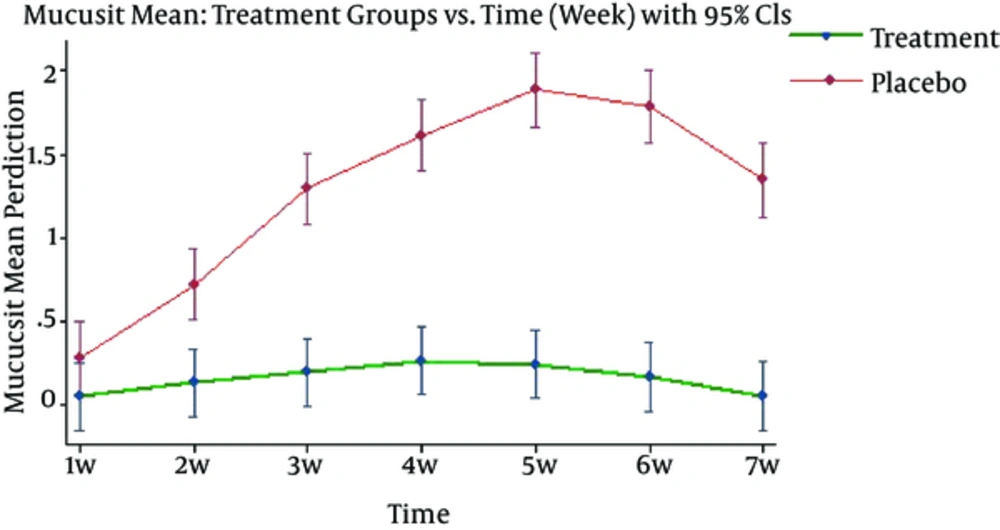
Generally, the differences of the severity of mucositis in all areas including upper and lower labia, upper and lower gingiva, dorsal and ventral surface of tongue, right and left buccal mucosa, soft and hard palate, floor of mouth and oropharynx were same between 2 groups, and from week 2, subjects in control group showed more severe mucositis than the experimental group (< 0.05). After analyzing data, using mixed model, it was found that drug effect, time effect, and time-drug interaction on average mucositis score are statistically significant (p < 0.0001) (Figure 2).
Since the time-drug interaction is significant, drug effect in any time, and vice versa, time effect in experimental and control group were analyzed. The average of mucositis severity showed significant difference between 2 groups so that mucositis score in control group was higher than experimental group in every weekly cutting (p < 0.0001) (Table 2).
| Week | Drug Group, Mean ± SE | Placebo Group, Mean ± SE* | p-Value |
|---|---|---|---|
| 1 | 0.048 ± 0.104 | 0.280 ± 0.109 | 0.1263 |
| 2 | 0.131 ± 0.104 | 0.719 ± 0.109 | 0.0001 |
| 3 | 0.194 ± 0.104 | 1.295 ± 0.109 | 0.0000 |
| 4 | 0.263 ± 0.104 | 1.613 ± 0.109 | 0.0000 |
| 5 | 0.243 ± 0.104 | 1.887 ± 0.112 | 0.0000 |
| 6 | 0.166 ± 0.104 | 1.787 ± 0.112 | 0.0000 |
| 7 | 0.055 ± 0.104 | 1.345 ± 0.112 | 0.0000 |
Moreover, time effect was significant only in control group (p < 0.0001) and in experimental group, there were no significant differences in mucositis score during the study (p = 0.2366). Therefore, not only the mucositis score was significantly lower in experimental group compared with control group, but also it was invariant during the study and showed no uptrend.
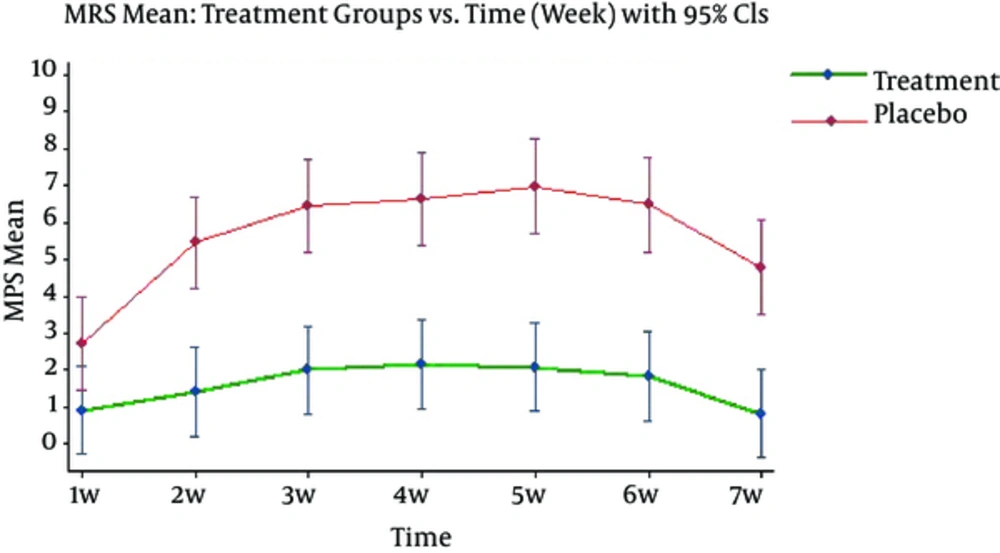
Drug effect and time effect (p < 0.0001) and time-drug interaction (p < 0.021) on average MPS were statistically significant.
Since the time-drug interaction is significant, drug effect in any time, and vice versa, time effect in experimental and control group were analyzed. The average of MPS showed significant difference between 2 groups (Figure 3) so that average MPS in control group is higher than experimental group in every weekly cutting (p < 0.0001) (Table 3).
| Week | Drug Group, Mean ± SE | Placebo Group, Mean ± SE* | p-Value |
|---|---|---|---|
| 1 | 0.916 ± 0.611 | 2.727 ± 0.639 | 0.0407 |
| 2 | 1.416 ± 0.611 | 5.454 ± 0.639 | 0.0000 |
| 3 | 2.000 ± 0.611 | 6.454 ± 0.639 | 0.0000 |
| 4 | 2.166 ± 0.611 | 6.636 ± 0.639 | 0.0000 |
| 5 | 2.083 ± 0.611 | 6.986 ± 0.659 | 0.0000 |
| 6 | 1.833 ± 0.611 | 6.486 ± 0.659 | 0.0000 |
| 7 | 0.833 ± 0.611 | 4.786 ± 0.659 | 0.0000 |
Likewise, time effect was significant only in control group (p < 0.0001) and in experimental group, there was no significant difference in average MPS during the time (p = 0.169). Therefore, not only the MPS was significantly lower in experimental group in comparison with control group, but also it was invariant during the study and showed no uptrend.
5. Discussion
Mucositis is the most common side effect of radiotherapy in patients with H and N cancer that reduces patients’ quality of life because of its complications. According to WHO, in patients with H and N cancer who received high doses of radiation, grade 3 and 4 mucositis reaches to 85%, but all patients get some degrees of mucositis (6).
A.digitata and M.sylvestris were described in Persian medicine as mucilaginous plants that can be used for their emollient, laxative, anti-inflammatory, and pain relieving properties (15, 16). The antioxidant (21), anti-inflammatory (22), and antimicrobial (23, 24) effects of these plants were investigated in the recent studies. Also, it was shown that A.digitata and M.sylvestris are mucilaginous plants that can be used to improve dry mouth (25).
The present study is the first study evaluating the efficacy of an herbal compound, containing these 2 herbs in prevention of radiation-induced mucositis in these patients compared with placebo. Mouth pain score was assessed by the patients, using visual analog scale and mucositis grade was evaluated by investigator, according to WHO scale in weekly visits.
The herbal compound showed beneficial effects in prevention of mucositis so that from week 1, the severity of mucositis and the average MPS were significantly lower in experimental group compared with control group; they were also invariant during the study and showed no uptrend, while using these 2 herbs showed no significant side effects in the patients.
The pharmacological effects of these herbs can be considered from 2 points of view. In new studies, effects of these herbs in decreasing irritations and inflammations of oral, pharyngeal, and gastric mucous (17), as well as antioxidant (18) and immunomodulatory effects (20) of them have been demonstrated. Moreover, anti-septic effects of M.sylvestris were studied (16, 19). Besides, it was mentioned in Persian medicine manuscripts that these 2 herbs can be used in decreasing mucosal and cutaneous inflammations (from mouth to intestines, ears, eyelids, and brain), and the reduction of pain and swelling of mucus membranes and have been used to heal the wounds, orally and topically (15, 16).
In conclusion, it is suggested that a compound drug containing A.digitata and M.sylvestris can be beneficial to prevent radiation-induced mucositis and decrease the severity and complications of this condition. It is important because there is no same drug for prevention or treatment of mucositis. The number of included patients was the main limitation of this study. It is suggested that these herbs can be surveyed in further studies with a larger sample size.
The results of this investigation support the efficacy of the herbal compound drug containing A.digitata and M.sylvestris for prevention of radiation-induced acute mucositis in patients with head and neck cancer.