1. Background
Cervical cancer is the second most common cancer to affect women over the world with 527,624 new cases and 265,672 deaths reports, annually (1). According to recently published data, each year, more than 400,000 cases may be diagnosed in the world comprising approximately 12% of the most commonly diagnosed caners among women (2). Cervical cancer is also one of the most common neoplasms among women both in low and middle-income countries (3). In other words, approximately, 90% of cervical cancer deaths occur in low and middle-income regions like Sub-Saharan Africa, Latin America, and the Caribbean (4). It is estimated that the incidence proportion of cervical neoplasm in Iran is 2.4 per 100,000 per year women (5).
Several risk factors may contribute in developing the disease such as early marriage, sexual relations before the age of 18, frequent marriage history, multiple pregnancies and childbirth, smoking, immunosuppressive diseases as well as low socioeconomic status, and genital infections like human papillomavirus (HPV), which is a sexually transmitted infection (6). Currently, Pap smear screening of cervical cancer has been one of the most successful public health measures over the last decade (3). In addition to the low cost, the test has high potential of early stage cervical cancer detection in women looking normal (7).
It has been reported that the Pap test can reduce the incidence rate and the mortality rate of cervical cancer by 79% and 70%, respectively (8). Thus, Pap smear screening exam may be considered as a cheap tool to early detection and primary prevention of cervical malignancies (9). Despite the significant success of the test in detecting cervical cancer, the participation rate in developing countries is only 5%, while in the high-income countries like the U.S., the corresponding proportion is about 90% (10). In Iran, several studies (11, 12) have also reported the low participation rate for the test. For instance, Farzaneh et al. in Ardabil, Iran indicated that 27.1% of the women referred to the comprehensive health services centers had the history of Pap smear uptake (11).
One of the reasons for the low participation rate of the test may be due to the lack of awareness about its importance. Therefore, informing the women about the importance and effectiveness of early diagnosis of cervical neoplasm could be important steps in promoting the participation rate at the national scale (13). However, before an early detection intervention for Pap smear uptake, there is a necessity for identifying the factors related to and the barriers correlated with the behavior.
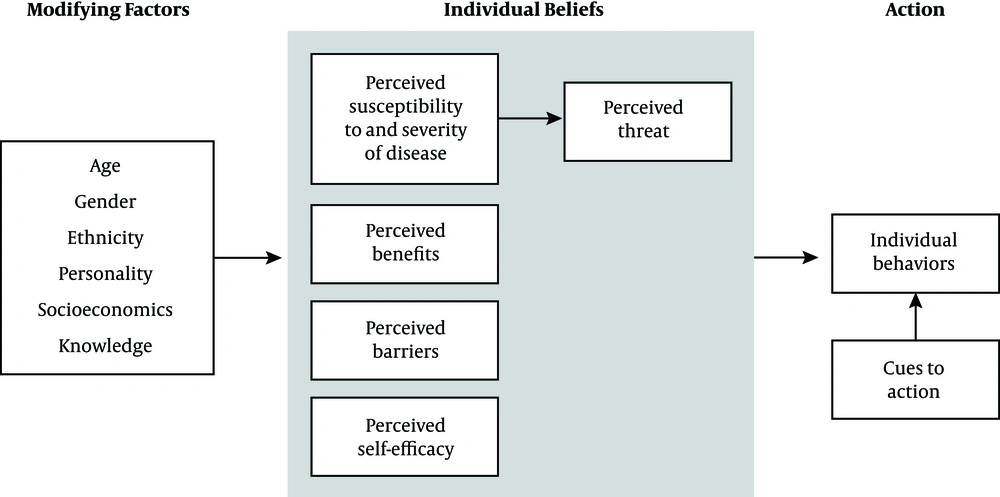
Since the theory-based interventions provide an appropriate structure to develop and have a guide for evaluation, they are more effective in influencing health-related behaviors compared to the non-theoretical approaches (14, 15). Researchers have applied some models for changing the health behaviors. Health belief model (HBM) (Figure 1) is one of the most appropriate models in the field of changing health behaviors. Public health services to forecast the health-promoting behaviors, such as uptake of screening programs. This model, as a comprehensive model, is based on people’s motivation for health action and focuses on the way that an individual percepts are motivated toward, and implementation a healthy behavior (16).
The HBM comprises several primary concepts that explain why people will take action to prevent, to be screened for, or to control illness conditions: (1) perceived susceptibility: refers to people’s beliefs about the possibility of having a disease or condition; (2) perceived Severity: people’s feelings about the seriousness of having an illness or leaving it untreated, which includes the assessment of possible clinical (like death, disability, and pain), and social complications (such as effects of the conditions on work, family life, etc.); (3) perceived benefits: refers to beliefs that the preventive behaviors are useful and effective in reducing the risk or seriousness of the impact; (4) perceived barriers: refers to beliefs about the tangible and psychological costs of the advised action that may act as impediments for undertaking recommended behaviors; (5) Self-efficacy: self-efficacy is defined as “the conviction that one can successfully executes the behavior required to produce the outcomes”; (6) cues to action: contributes to the person’s perception of the threat. Cues to action can be internal (e.g., bodily state or symptom) or external (e.g., reminder about doctor’s appointment) (17).
HBM has been widely applied as a theoretical framework to explain health promoting behaviors and to guide the researchers in their health behavior interventions (18). Its reliability and validity has been previously approved to identify the beliefs in the field of cervical cancer prevention (19).
2. Objectives
This study was performed to identify the determinants of Pap smear screening (PSS) behavior among a group of rural women in Tabriz, Iran, using the HBM model. Identifying the effective factors on PSS may be useful in developing the interventional plans aiming at the promotion of cervical cancer screening behavior.
3. Methods
3.1. Sampling
This cross sectional study was conducted during September to November 2017 among the rural women referred to the rural health centers in Tabriz, East Azerbaijan province, Iran. Multistage cluster sampling was employed to recruit 220 participants of the study. Two comprehensive health centers were randomly selected out of 5 and the women at the 2 centers entered the study based on their records. The respondents were invited by phone call to participate in the study. When attending the health center, the participants were informed about the research objectives and provided with written informed consent to be signed. Then, the questionnaires were completed in a consultation room in the health center. The women were interviewed to complete the HBM-based questionnaire. Due to nature of the study questions and regarding the culture of the study population, all the interviews were conducted by a trained female interviewer to make participants feel comfort. The inclusion criteria were the rural women, who were not pregnant and had one or more year (s) of espoused life and were consented for participation in this research.
3.2. Data Collection
A reliable and valid HBM-based questionnaire was used for data collection (20). This questionnaire was translated into Persian by Karimy et al. to investigate the HBM-based cognitive constructs that relate to Pap smear test in women, who were referred to health centers. In a study carried out by Karimi et al. the Cronbach’s alpha was 0.82 for the HBM-based questionnaire and 0.85 for the knowledge. In the current study, the Cronbach’s alpha coefficient was 0.76 for the HBM-based and 0.70 for the knowledge questionnaires. The items of the questionnaire were demographic data, comprised age, education level (illiterate/elementary, high school/diploma, university), economic status of the family (weak, fair, good), and history of urinary infection (yes/no). The knowledge scale, which had 12 items, was applied to assess the knowledge of the participants about signs and symptoms of the cervical neoplasm, its severity, and the preventive behaviors. As an example: “Early marriage (at the ages below 17) increases the risk of developing cervical cancer”. The answers for each item were yes (2), I don’t know (1), and no (0). A 5-item scale was used to measure the perceived susceptibility towards cervical cancer. As an example: “I am worried of being diagnosed with cervical cancer”. The perceived severity of the cervical cancer was examined by a 5-item scale, one of which, as an example, was: “The name of cervical cancer causes fear and panic in my mind”. There was a 5-point Likert scale for the items of the perceived susceptibility and perceived severity scales ranging from 1 to 5 (1 = totally disagree through 5 = totally agree). Higher scores indicated more susceptibility and severity towards the cervical cancer.
The perceived barriers and perceived benefits of conducting PSS included 12 items (6 items for each). Two example items of the perceived benefits and perceived barriers were “Having a Pap test will increase the chance of early diagnosis of a possible tumor in my cervix” and “I am too busy to find enough time to go for Pap smear test”, respectively. The scoring system of the scales of perceived benefits and perceived barriers was like the perceived susceptibility and severity, as described above. Higher scores on the benefits and lower scores on the barriers were desired.
The scale of self-efficacy to go Pap smear test included 10 items. “I am confident that I can encounter with unexpected problems, effectively” is an example of these items. In this scale, the answers were on a 4-point Likert scale ranging from 1 to 4 (1 = totally confident through 4 = totally unconfident). Higher scores meant more self-efficacy.
Finally, performing the Pap test was measured, using 1 question: “Have you had a Pap smear test in the previous 3 years?” The answer should be yes (1) or no (0).
3.3. Analysis
Data were coded numerically and entered into statistical package for social sciences (SPSS) software version 20 for windows. Summary statistics and frequency distributions were applied to describe and interpret the data. Possible differences in HBM constructs by the demographic variables were examined by One-way ANOVA and the independent samples t test. The associations between HBM constructs and the PSS behavior were analyzed, applying Pearson correlation coefficient test. In addition, logistic regression model with Enter method was used to explain the differences in PSS behavior by the HBM constructs. Shapiro-wilk test was applied to check the normality of the data with 0.05 level of significance. STATA 11 software was applied to analyze the data.
4. Results
The average age, at which the respondents began cervical cancer, was 31.25 ± 8.81 years old. Almost all participants were housewives (98.6%). Regarding the educational level of the participants, 99 (45.0%) of the subjects were illiterate, 72 (32.7%) had elementary, high schools, and diploma degrees, and 49 (22.3%) had academic education. Demographic characteristics of the participants are demonstrated in Table 1, divided by history of Pap smear test.
| Variables | Having Pap Test in Previous 3 Years | Not Having Pap Test in Previous 3 Years | P Valueb |
|---|---|---|---|
| Age groups | 0.001 | ||
| ≥ 25 | 54 (10.2) | 10 (44.3) | |
| 26 to 35 | 40 (48.0) | 47 (32.8) | |
| 36 to 45 | 21 (36.7) | 36 (17.2) | |
| 46 ≤ | 7 (5.5) | 5 (5.7) | |
| Level of education | 0.806 | ||
| Illiterate and elementary | 53 (43.4) | 46 (46.9) | |
| High school | 42 (34.4) | 30 (30.6) | |
| Diploma | 23 (18.9) | 17 (17.3) | |
| Bachelor | 4 (3.3) | 5 (5.1) | |
| History of urinary infection | 0.001 | ||
| Yes | 27 (22.1) | 43 (43.9) | |
| No | 95 (77.9) | 55 (56.1) | |
| Economic status of the family | 0.060 | ||
| Good | 32 (28.7) | 16 (16.3) | |
| Fair | 68 (55.7) | 63 (64.3) | |
| Poor | 19 (15.6) | 19 (19.4) |
aValues are expressed as No. (%).
bP value based on chi-square test.
As it can be seen in Table 2, there was a statistically significant association assuming the condition of hypothesis H0 a knowledge of rural women, who had and those who did not have a history of PSS in the last 3 years. Moreover, the score of perceived benefits of PSS was higher among those participants, who had a history of PSS in last 3 years (P = 0.001).
| Variables | Mean ± SD | Mean Difference (Std Error) | P Valuea |
|---|---|---|---|
| Knowledge | -1.92 (0.77) | 0.014 | |
| No | 25.50 ± 6.45 | ||
| Yes | 27.42 ± 5.06 | ||
| Perceived susceptibility | 0.30 (0.64) | 0.637 | |
| No | 15.22 ± 5.53 | ||
| Yes | 14.91 ± 4.03 | ||
| Perceived severity | - 0.65 (0.62) | 0.297 | |
| No | 11.73 ± 5.06 | ||
| Yes | 12.39 ± 4.26 | ||
| Perceived benefits | -3.48 (0.61) | 0.001 | |
| No | 9.43 ± 4.08 | ||
| Yes | 12.92 ± 4.87 | ||
| Perceived barriers | 1.25 (0.80) | 0.121 | |
| No | 20.81 ± 5.98 | ||
| Yes | 19.55 ± 5.94 | ||
| Perceived self-efficacy | 0.40 (0.90) | 0.654 | |
| No | 30.43 ± 7.10 | ||
| Yes | 30.00 ± 6.34 |
aP value was calculated based on independent t test.
The distribution of dependent variable followed the binominal distribution (P = 0.55) and the independent quantitative variables had a linear relationship with the logit of the dependent variables. The fitness of the model was examined by Hosmer-Lemeshow test based on the observed and expected cases in chi-square. The model was fit (P = 0.109). To assess the power of the model in classification of the subjects in categories of the dependent variable and the predictability of the model, the classification statistics after logistic was applied. The validity of the model was estimated 69.3%, which is good.
The results of the univariate analysis showed that the variables of awareness, perceived benefits, and age had significant correlation with performing Pap-smear test (P < 0.05).
According to the multivariable analysis shown in Table 3, the odds ratio of age = 0.94 (0.91 - 0.98) was statistically significant, so that with one unit increase in age, the odds of cervical cancer screening behavior decreased 6%. Furthermore, the odds ratio of perceived benefits was 1.18 (1.08 - 1.27) i.e. with one unit increase in perceived benefits, odds of cervical cancer screening behavior increased 18%.
| Variables | Univariable Model | Multivariable Model | ||
|---|---|---|---|---|
| OR | OR (95% CI) | OR | OR (95% CI) | |
| Knowledge | 1.06 | 1.01 - 1.12 | 1.01 | 0.95 - 1.06 |
| Perceived susceptibility | 0.98 | 0.93 - 1.04 | - | - |
| Perceived severity | 1.03 | 0.97 - 1.09 | - | - |
| Perceived benefits | 1.20 | 1.12 - 1.30 | 1.18 | 1.08 to 1.27 |
| Perceived barriers | 0.96 | 0.92 - 1.01 | - | - |
| Perceived self-efficacy | 1.01 | 0.96 - 1.06 | - | - |
| Age | 0.93 | 0.90 - 0.96 | 0.94 | 0.91 to 0.98 |
Nagelkerke R square for the multivariate model was estimated to be 0.231. So, the variables entered in the multivariate model predicted 23.1% of the pap-smear screening behavior of the participants.
Questions related to barriers of screening Pap test are shown in Table 4. The most important barriers to perform Pap test among the women were “I am in doubt with the efficacy of Pap smear test in detecting cervical cancer” (29.5%), and “I am afraid of being diagnosed with cervical cancer” (29.1%), respectively.
| Variables | Totally Agree | Agree | No Idea | Totally Disagree | Disagree |
|---|---|---|---|---|---|
| I hate such an examination and sampling procedure | 38 (17.3) | 44 (20.0) | 56 (25.5) | 50 (22.7) | 32 (14.5) |
| I am too busy to go for having Pap smear test | 22 (10.0) | 21 (9.5) | 59 (26.8) | 69 (31.4) | 49 (22.3) |
| If I would have cervical cancer, I prefer not to be aware of. | 27 (12.3) | 17 (7.7) | 55 (25.0) | 63 (28.6) | 58 (26.4) |
| Such cancers are the results of fate | 34 (15.5) | 34 (15.5) | 43 (19.5) | 60 (27.3) | 49 (22.3) |
| I am afraid of being diagnosed with cervical cancer | 25 (11.4) | 21 (9.5) | 48 (21.8) | 62 (28.2) | 64 (29.1) |
| I am in doubt with the efficacy of Pap smear test in detecting cervical cancer | 20 (9.1) | 30 (13.6) | 65 (29.5) | 62 (28.1) | 43 (19.5) |
aValues are expressed as No. (%).
5. Discussion
Because of the importance of the participation of women in CCS, this study investigated the determinant factors of health behaviors of rural women of Tabriz, Iran in such program. The study found that 55% of the rural women have participated in PSS in the last 3 years. Lofters et al. reported it 53.1% (21) in Canada and Sauer reported it 90.5 in US (22), 42% in California in the past 1 year, and 72% in the last 2 years (23). The role of rural environment should be considered when talking in this regard. The participation rate of rural women in this study was higher than Kurdish women west of Iran in a study by Aminisani et al. (32%) (24). It seems that women with specific characteristics had higher participation in CCS: family history of cervical cancer, minor genial infections, high socioeconomic level, higher education (women or their spouses), and universal coverage of primary healthcare in rural areas of Iran (25).
There was a reverse correlation between having CCS and the age of the participants, so that the higher the age of women, the less their intention to perform Pap smear. The study of Silva in urban women of Brazil reported the menopause as the reason for the fall of Pap smear participation (26). Schlichte and Guidry also found that women of higher ages report the test as unnecessary (27). In Canada, the lower participation of women in Pap test was correlated with not being classified in age group of 35 to 49 years (28). In this study, it seems that the higher health literacy of younger women and being in sexually active ages are the reasons for higher participation in CCS. Although women of older cohorts showed less CCS behavior than the younger, it might be due to the cross sectional nature of the study and the cohort effect.
A correlation was observed between the history of urinary infection and the CCS behavior, so that those women with the history of urinary infection had more intention to perform Pap smear. Babazadeh et al. stated the perceived severity of the disease (29) and Karimy et al. (20) stated the fear of disease consequences as the reason for the higher participation of women with urinary infection in the PSS. It seems that these women are more sensitive in follow-up and referral to midwifery services. Yet, the fact that women with the history of urinary infection had more participation in CCS might be, to some extent, due to focus of the health service providers on women in sexually active ages. Moreover, the proper health behavior of these women, compared to those who had not history of urinary infection, shows a good care high risk people. It is also needed to be considered that the history of urinary infection can act as a bias by indication because urinary infection is an indication of CCS.
In this study, no statistically significant difference was observed on the number of Pap smear tests in the last 3 years in terms of economic status of the women. It seems that this finding is related to the nature of the study population, which is consisted of the rural women; because the income inequality within the rural population is low (30). Yet, other studies have reported the effect of economic factors on PSS behavior. A study in Vietnam reported high cost of the PSS and lack of health insurance among the reasons of avoiding PSS in the last 12 months (31). In Canada, the migrant women had lower rate of PSS and those women in lower social classes had lower health literacy, lower social capital, and non-scientific traditional beliefs (32). Thus, it is recommended to put more emphasize on PSS in middle and lower social classes in rural population. Furthermore, according to the Hill’s criteria for causation, the socioeconomic status affects the PSS behavior by temporal sequence principles.
The findings of this study showed that women, who had participated in PSS, had higher awareness than those who had not. A study conducted by Allahverdipour and Emami in Iran reported that one-third of the women had low awareness on cervical cancer (33). Another study in China reported that only 32% of the participants had a reasonable knowledge on cervical cancer (34). The low awareness of the women in most studies conducted in Iran might be due to the lack of a comprehensive educational program for women on cervical cancer and the PSS. Moreover, cultural differences between the societies can be affective on the level of awareness on cervical cancer. A study in Qatar reported women with diagnosed cervical cancer, employed, 15 years and more of married life, academic education, and more than 3 birth giving were most likely to participate in PSS (35). Since the rural women in Iran usually marry in lower ages and had little opportunity for higher education and regarding the fact that 47% of the participants in this study were illiterate or with low education, there is a necessity for educations on CCS, which should be appropriate for rural culture. Education of the spouses and using the health providers might be other effective interventions (36).
The average score of perceived s of PSS had a significant difference between women, who had and those who did not have the history of CCS. Based on this finding, we can recommend intervention measures for increasing the awareness of rural women about the benefits of PSS to increase their participation in the program. The “perceived benefits” was the only construct of the health belief model that was significant among the rural women. Majority of these women had low age and education. Thus, they might not have an accurate understanding of susceptibility and severity of the cervical cancer and in their opinion, the incidence of the cancer is mostly chance-dependent. This means that the behaviors of those women, who were familiar with mechanism and the risk factors of cervical cancer, were more predictable by the health beliefs model. In a study in urban Iran, using the health belief model, the awareness of the women, perceived severity, perceived benefits, perceived barriers, and perceived self-efficacy were the predictors of CCS behavior (37). The fact that other constructs of the model were not significant predictors of the behavior of the rural women might also be due to their little knowledge about the Pap smear. If then, the health workers who promote the Pap smear should rearrange their attempts on rural population.
The situation of the constructs of the health belief model was not good in this study, so that the average scores of the perceived susceptibility and perceived severity among women who had the history of PSS in the last 3 years were higher than those women who did not have. This finding is in line with the study of Allahverdipour and Emami in which 24.9% of the participants were in a good situation on perceived susceptibility and 32.8% in a good situation on perceived severity (33). In addition, in the study of Allahverdipour, the perceived benefits and barriers in 47% of the cases were in a poor situation (33). The perceived barriers in the study of Allahverdipour and the perceived benefits and barriers were the predictors of PSS (33).
The “perceived benefits” in both univariate and multivariate models was a predictor of CCS behavior. In the final model, which showed closer results to the reality, the perceived benefits was the best predictor of PSS in rural women. A negative correlation was also observed between age and the perceived benefits in this study. Women in lower ages usually perceive lower benefits for PSS due to marriage in lower age, lower education, and health literacy. In the study of Hope et al., the perceived barriers, perceived severity, and awareness were the predictors of PSS (38). Another study by Costa et al. reported the perceived benefits and barriers as predictors of PSS behavior among women (39). In a study carried out by Miri et al. in Birjand, Iran, they demonstrated that the perceived benefits (β = 0.17, P = 0.01), the perceived barriers (β = -0.19, P = 0.01), and the perceived self-efficacy (β = 0.10, P = 0.01) have direct and significant effects on Pap smear behavior. The perceived threat (β = 0.002, P = 0.99) has no significant direct effect on Pap smear behavior (40).
5.1. Conclusions
The use of health belief model in identifying the predictors of PSS among rural women was successful. The awareness and the perceived benefits of the PSS were the most important predictors of CCS behavior. Thus, the health service providers should focus on increasing the awareness of the rural women on cervical cancer, PSS, and its benefits. The barriers of the PSS should be removed and the misconceptions of the women should be resolved. Based on the findings of this study, to increase the participation rate of the rural women in PSS program, the health workers should explain the mechanism of cervical cancer and highlight the benefits of the PSS in its early detection. Yet, other constructs of the model such as susceptibility and severity may not be ignored in the education efforts.
5.2. Limitations
One of the limitations of this study is the self-report nature of the participants’ data, which might result in over-reporting the PSS. Another point to consider is that all the participants of this study were from the Turkic ethnicity, which may limit the generalizability of the results to other ethnic groups. The third point is the possibility of selection bias. Yet, the strength of the study is studying women with no history of hysterectomy.