1. Background
Gestational trophoblastic disease (GTD) includes a group of pregnancy-related disorders, arising from the premalignant disorders of complete and partial hydatidiform mole, the malignant disorders of invasive mole, choriocarcinoma, and the rare placental-site trophoblastic tumor. Gestational trophoblastic neoplasia (GTN) is used to describe these malignant forms and it was recommended by international federation of obstetrics and gynecology (FIGO) for patients, whose serum beta-human Chorionic Gonadotropin (Beta-hCG) level failed to regress in the absence of a normal pregnancy (1, 2). Hydatidiform mole is mostly diagnosed in the first trimester of pregnancy during routine pregnancy tests; however, its clinical signs and symptoms are rarely observed at present. The most common incidence of GTN is in molar pregnancy, but the malignancy may be observed after any pregnancy (3, 4). Research studies in complete and partial molar pregnancies showed that approximately 15% and 1% to 5% of the women are under the risk of GTN, respectively (5, 6).
In general, this neoplasia is stated by several criteria; β-hCG levels is not reduced during 4 consecutive measurements, the β-hCG hormone serum titer increases over 3 consecutive weeks, β-hCG serum titer is detectable 6 months after the evacuation of molar pregnancy and, finally, the histological diagnosis of choriocarcinoma (7).
Commonly suction curettage is the typical method of evacuation of the molar tissue when molar pregnancy is supposed. After the molar evacuation, all individuals do not progress towards spontaneous recovery and some of them suffer from malignant problems (eg. GTN) and require chemotherapy. In this context, identifying an appropriate diagnostic indicators for the early detection of these malignancies is of great importance (4, 8).
Currently, there is not any definite diagnostic marker for early prediction of neoplasia, while all gestational trophoblastic diseases produce human chorionic gonadotropin (hCG). Therefore, as an standard method in the diagnosis of trophoblastic disease, serum β-hCG titer is followed during the weeks after molar pregnancy evacuation (9). In the previous decade, a number of studies have been conducted to find some indicators for early prediction of GTN. For instance, some research indicated that the ratio of β-hCG concentration before and after molar pregnancy or the ratios of hCGα- and β-subunits may lead to better early prediction of GTN (10-12). In addition, in another study, the researchers used the slope of linear regression between logβ-hCG and time (in week) and regression curve for this purpose (13-15).
Although most of the researchers in this field have measured the β-hCG repeatedly after mole evacuation surgery, they have assessed only the cross sectional effect of each measurement on the incidence of GTN. Regarding this shortage in previous studies as well as the importance of early detection of GTN in women with molar pregnancy, we decided to use more advanced statistical models (shared random effects) to evaluate the relationship between repeated measures of β-hCG (as a longitudinal indicators) and the incidence of GTN. To do this, we used the data from a historical cohort study conducted in women with hydatidiform mole according to their pathological results referred to the educational and health care centers affiliated to Shahid Beheshti University of Medical Sciences between 2003 and 2013.
The principal objective of the present study was to find the sensitivity and specificity of repeated β-hCGs during 3 weeks after molar evacuation surgery in predicting GTN.
2. Methods
2.1. Patients
In this study, the documents of all pregnant women, who were referred to the educational and health care centers affiliated to Shahid Beheshti University of Medical Sciences between 2003 and 2013 and diagnosed with hydatidiform mole, according to their pathological results, were investigated. Informed consent was obtained from all individual participants of the study. After primary assessments, 221 cases from 98 658 births of molar pregnancy with complete and partial mole who had at least 4-titer β-hCG records, were identified and included in the study. Among 221 patients, 20 were excluded from the study (9 for receiving chemoprophylaxis, 3 women because of initial treatment by hysterectomy, and 8 women because of incomplete document). Of the remained 201 women, 31 (15%) had GTN, and the remaining cases experienced normal hormone level in serum naturally during follow-up. In the current study, the first titer of β-hCG hormone was measured and documented at most 48 hours after the molar pregnancy evacuation (16). The follow-up process for patients was in a way that their titer of β-hCG hormone was measured weekly until 3 consecutive normal titers were attained and it continued monthly for 6 months. All procedures performed in this study involving human participants were in accordance with the ethics committee of Shahid Beheshti University of Medical Sciences and its later amendments or comparable ethical standards.
The repeated measures of log-transformed β-hCG concentrations at 4 consecutive time points (days 0, 7, 14, and 21) were considered the predictor of GTN in women with molar pregnancy. Sensitive and specific radioimmunoassays (RIAs) were used for all β-hCG measurements based on polyclonal antibody raised in rabbits. More details about the laboratory assessments and RIAs of β-hCG can be found elsewhere (17). In addition, the main result of the study was the presence of GTN during 6 month follow-up (1 = presence of GTN, 0 = absence of GTN).
2.2. Statistical Analysis
In the present study, we used a shared random effects (SRE) model to evaluate the power of longitudinal β-hCG concentration to predict GTN (18). This method can be simplified to a two-step procedure under a Gaussian random effects assumption. In the first step, a linear mixed model was fitted to the data to solve the longitudinal (repeated) biomarker combination problem, using the following equation:

where X𝑖 and Z𝑖 are the design matrices of the fixed and random effects, θ is the vector of fixed effects parameters, b𝑖 ∼ MVN (0, ∆) is the vector of random terms, and τ𝑖 ∼ MVN (0, ∑i) is the vector of residuals. In this study, we used the following random intercept-random slope model for the repeated β-hCGs (1):

where t𝑖j is the time of measurement for 𝑖th participant at 𝑗th visit.
In the next step, a multiple logistic regression model was fitted to the disease outcome (the presence or absence of GTN), using the predicted random terms (estimated b0i and b1i) from the model in the first step as covariates. Then, the binary outcome variable Di can be linked to the longitudinal process as:

where α is the model intercept, and βks are the regression slopes related to the estimated subject-specific random terms b0i and b1i. The goal for fitting this logistic model was estimating the probability of GTN for each subject under study, that is p (Di = 1). The estimated probabilities were utilized to assess the predictive power of β-hCG values, using the area under ROC curve (AUC) index. The lmer package in the statistical software R (version 2.15.3) was used for fitting the described models.
3. Results
The registered information of 201 women showed that vaginal bleeding was the main cause of referring to the hospitals under study (45.4%). In addition, 44% of cases were diagnosed via periodic ultrasound for pregnancy. The mean age (SD) of women was 26.7 (6.7) years and approximately 6% of the patients were immigrant Afghan women living in Iran. Among them, 171 (85.1%) had spontaneous remission, and GTN was detected in 30 cases (14.9%). Table 1 compares the characteristics of women with and without GTN.
| Characteristic | Category | GTN | |
|---|---|---|---|
| Presence | Absence | ||
| Mole type | Complete | 25 (13.8%) | 156 (86.2%) |
| Partial | 5 (25%) | 15 (75%) | |
| Race | Iranian | 27 (14.2%) | 163 (85.8%) |
| Afghan | 3 (27.3%) | 8 (72.7%) | |
| Abortion history | Yes | 9 (25%) | 27 (75%) |
| No | 21 (12.7%) | 144 (87.3%) | |
| Parity | 0 | 19 (63.3%) | 84 (49.1) |
| 1 | 6 (20%) | 58 (33.9) | |
| Above 2 | 5 (16.7%) | 29 (17%) | |
| Uterine height, mean ± SD | - | 10.62 ± 3.74 | 10.18 ± 3.415 |
| Mother’s age, mean ± SD | - | 26.43 ± 5.71 | 26.94 ± 6.7 |
| Gestational age, mean ± SD | - | 9.59 ± 2.61 | 10.13 ± 2.67 |
aValues are expressed as No. (%) unless otherwise indicated.
Table 2 shows the descriptive statistics for β-hCG levels as well as P values for comparing the mean β-hCGs between women with and without GTN at different time points. The reported P values indicated that the difference between mean β-hCG values in 2 groups were not significant at the first and second time points (days 0, 7), but significant differences could be observed in days 14 and 21. The results suggested that the values of this biomarker in weeks 2 and 3 had more predictive power for discriminating women with and without GTN.
| Day | GTN, mean ± SD | Pa | |
|---|---|---|---|
| Positive | Negative | ||
| 0 | 10.45 ± 1.44 | 10.04 ± 1.49 | 0.164 |
| 7 | 9.67 ± 1.30 | 9.13 ± 1.51 | 0.066 |
| 14 | 9.12 ± 1.23 | 7.20 ± 1.25 | 0.000 |
| 21 | 8.81 ± 1.41 | 6.55 ± 1.18 | 0.000 |
aFrom independent samples t test.
Regarding these results, we, firstly, fitted the SRE model with β-hCG levels in weeks 2 and 3 (model 1); then, we fitted this model with β-hCG levels in weeks 0, 1, 2, and 3 (model 2). Next, the estimated random terms from models 1 and 2 were used in the above-mentioned logistic model to obtain the probability of GTN for each case under study. Finally, these estimated probabilities were utilized to predict GTN in the ROC curve analysis.
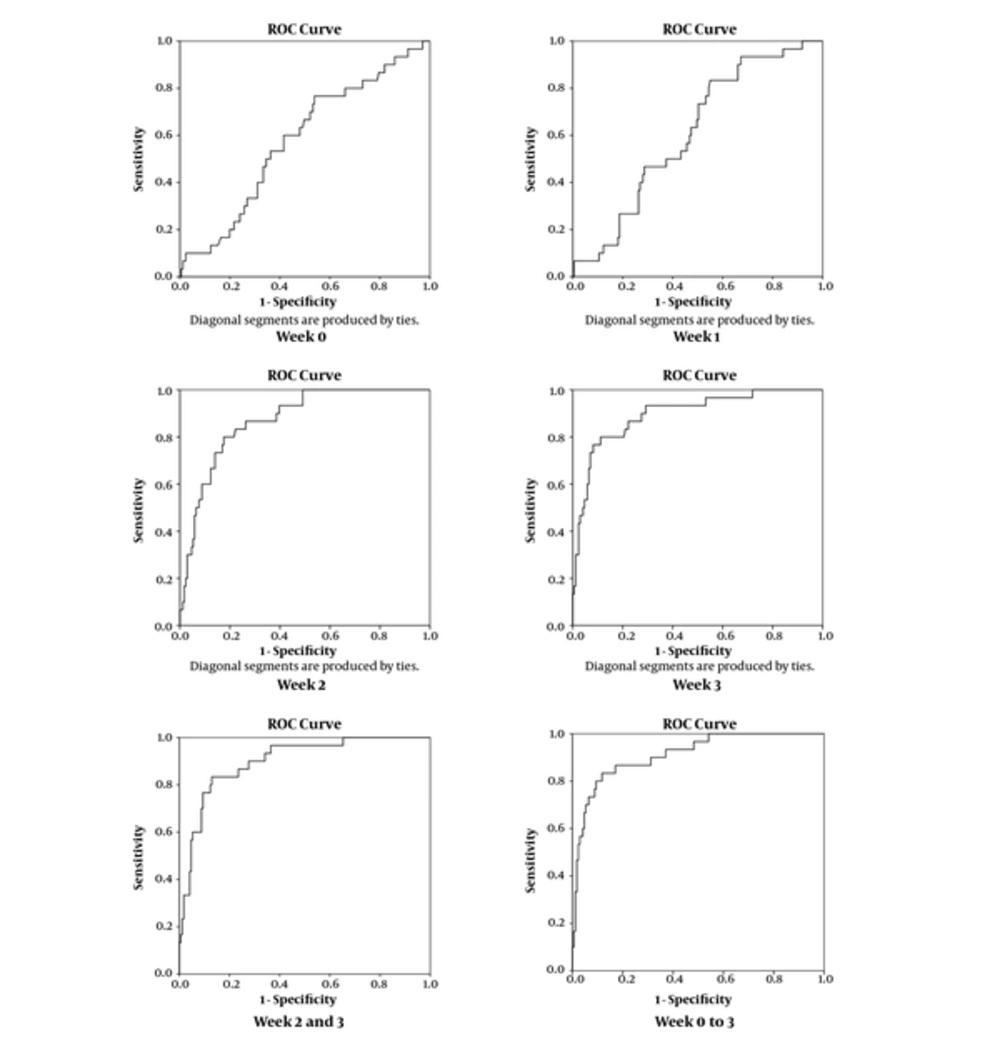
Table 3 displays the obtained sensitivity, specificity, and AUC from the ROC curve analysis for the estimated probabilities based on models 1 and 2 as well as the power of β-hCG levels to predict GTN, separately in weeks 0, 1, 2, and 3. The obtained indices (sensitivity, specificity, and AUC), based on the best cut-off points for the estimated probabilities, shows that the model 2, which uses the β-hCG levels from all weeks, had the best power to predict GTN (AUC = 91.2%). However, the predictive power of β-hCG levels separately in weeks 2, and 3 in addition to model 1, which uses the β-hCG levels in weeks 2 and 3, seems to be nearly close to the obtained findings from model 2 (AUC = 87.3%, 89.7%, and 89.9%, respectively). Figure 1 shows the obtained ROC curves.
| Week | Sensitivity | Specificity | AUC |
|---|---|---|---|
| 0 | 56.7% | 59.0% | 58.0% |
| 1 | 70.0% | 50.0% | 61.0% |
| 2 | 86.7% | 74.1% | 87.3% |
| 3 | 86.7% | 78.2% | 89.7% |
| 2, 3 (Model 1) | 86.7% | 72.4% | 89.9% |
| 0, 1, 2, 3 (Model 2) | 86.7% | 83.0% | 91.2% |
4. Discussion
The present study aimed at evaluating the trend of β-hCG concentration 21 days after molar evacuation as well as investigating the power of this marker in the early prediction of individuals with GTN. In general, findings of this study revealed that the trend of β-hCG concentration during 3 consecutive weeks after molar evacuation in women with hydatidiform mole may be considered as an appropriate marker for predicting GTN. In other words, the obtained results showed that more than 90% of women with GTN could be classified correctly, using the 21 days trend of β-hCG concentration after mole evacuation. The early detection of GTN can help the physicians start the treatment methods at an early stage and prevent the consequences of this disease, such as metastasis and death. Previous research in this field also stated that the higher concentrations of β-hCG compared with other markers, such as hCG-α in addition to increased levels of free-β-hCG can be considered a signal for GTN in women with molar pregnancy (10, 19, 20).
In this study, the difference between mean β-hCG values in women with and without GTN was significant at weeks 2 and 3 after mole evacuation. This finding suggests that the β-hCG levels in weeks 2 and 3 had more predictive power for discriminating these 2 groups of women. In a study conducted by Kang et al., it was reported that the median hCG level 2 weeks after evacuation in the patients with GTN was significantly higher than the remission group. They also showed that the ratio of pre-evacuation hCG to hCG 2 weeks after evacuation was the best predictive factor, according to the ROC curve, with AUC of 77.3% (12). Likewise, other cross sectional studies in this field suggested different ratios of hCG levels as appropriate predictor for GTN in patients with molar pregnancy (21). For instance, in a study conducted by Van Trommel et al., they calculated hCG ratios from serum hCG concentrations for 204 patients with and without persistent trophoblastic disease (PTD). The hCG ratios obtained in week 1, 3, and 5 after evacuation identified, respectively, 20%, 52%, and 79% of patients with PTD (11). However, the present study gives more accurate discrimination (above 90%), using the three-week trend of β-hCG levels. Furthermore, the slope of the linear regression line was used as the marker for prediction of GTN in some other studies. Kim et al. showed that the hCG regression rate (hCG divided by initial hCG) could predict the GTN with a sensitivity of 48.0% and specificity of 89.5% (AUC = 0.759) in the second week after evacuation. In their study, only the patients with an initial hCG level of more than 100 000 IU/L were investigated (22). In another study, among 113 patients with at least 3 log-transformed serum hCG values from day 7 to 28 after evacuation, AUC of 90% and 84.4% were, respectively, reported, using the slopes of the hCG and β-hCG linear regression lines. They could correctly classify 69.0% of patients with GTN and 97.5% of patients without GTN within 28 days after evacuation, using the slope of free β-hCG (23). Compared with these findings, the modeling approach used in the present study resulted in higher sensitivity within a shorter time (21 days).
Reviewing the previously published articles in this field tells us that almost all research studies have applied rather simple statistical methods, such as univariate tests, simple regression models, and straight forward ROC analysis to investigate the predictive power of biomarkers in early detection of GTN. For instance, Shigematsu et al. used a stepwise piecewise linear regression model to establish a normal curve for discriminating patients with PTD from uneventful moles (14). They concluded that this normal regression curve is useful for discriminating PTD from uneventful moles more quickly than recommended curve by FIGO. Utilizing normal β-hCG regression curves is a common approach for predicting GTN in most of the articles (13-15, 24). Using the repeated measures of β-hCG concentrations as the predictor of GTN in women with molar pregnancy was the main difference between the current study and research studies conducted by others. In other words, we considered the β-hCG values longitudinally, but other research studies considered this biomarker in a cross sectional manner. The repeated measures of an outcome contain more information compared with a single observation of this outcome. Therefore, we expect that our statistical approach that accounts for this additional information lead to more accurate results than others. One of the most important strength points of our modeling approach is that using this model, one can include individual covariates (such as demographic characteristics, laboratory indices. etc.) to improve the accuracy of prediction. Because of the incomplete registration of important covariates in our sample files, as one of the limitations of the present study, we did not include these covariates into the model. However, if one can include significant covariates into this model, higher values of accuracy (sensitivity and specificity of about 100%) are expected.
4.1. Conclusions
To conclude, the results of this study indicate that the two-week β-hCG concentration (longitudinal classifier) in weeks 2 and 3 can predict over 80% of patients who get GTN after molar evacuation. Besides, over 90% of patients who have advanced getting GTN can be correctly identified if we apply all their measurements in the model (days 0, 7, 14, and 21). Therefore, monitoring the three-week trend of this marker is recommended for the early detection of this malignancy in women with molar pregnancy.