1. Background
Esophageal cancer (EC) is a common cancer worldwide. It is responsible for the 6th cancer-related death (1, 2). Considerably, high incidence rates have been identified in the region termed “Asian Esophageal Cancer Belt”, stretching from north of Turkey, north provinces of Iran through Turkmenistan, central Asian countries such as China, and north into Russia. The reasons for this geographic variability are unknown (3, 4).
Esophageal cancer histologically has two subtypes, namely esophageal adenocarcinoma (EAC), and esophageal squamous cell carcinoma (ESCC). Albeit both subtypes are seen in the esophageal tissue, the distribution and etiology of them vary. The ESCC is the most prevalent subtype of EC in developing countries (2, 5).
Esophageal cancer is usually diagnosed at late stages when invasive tumors have developed offense to peripheral tissues and lead to dysphagia. Although there are improved therapies such as surgery and chemoradiation, EC has a poor prognosis (5-year survival equals 19%). Nowadays, there are no screening methods for the recognition of EC at the early stages. Therefore, the identification of main etiology and prevention of EC is important to decrease EC burden (5).
The main etiology of EC remains undetermined. Epidemiologic studies have suggested that tobacco, opium, alcohol consuming, hot tea drinking, food carcinogens, nutritional deficiencies, lifestyle factors, some chemicals such as polycyclic aromatic hydrocarbons, genetic factors, and infectious agents may have a role in the pathogenesis of EC (3, 5, 6).
In the infectious etiology of EC, human papillomavirus (HPV) related morphological changes were first seen in both benign and malignant esophageal tissues in 1982 (7). Then, accumulating laboratory and epidemiological evidence suggests an important role for HPV in ESCC (3, 8).
HPV genome is a circular double-stranded deoxyribonucleic acid (dsDNA) containing early (E1 through E7) open reading frames (ORFs) encoding viral early (nonstructural) proteins and late ORFs (L1, L2) encoding viral structural proteins. The carcinogenic mechanism of HPV is related to E6 and E7 oncoproteins, which change cell regulatory proteins such as p53 and Rb and cause abnormal proliferation of host cell and malignancy. In addition, in malignant tissues, the viral genome integrates into the cell chromosome and the integration disrupts E2 and L ORFs; therefore, the expression of viral regulatory protein (E2) decreases. Decreasing E2 results in the elimination of E2 suppression effect on the promoters of the viral oncoproteins (E6 and E7); therefore, the expression of E6 and E7 increases (8).
According to the sequence of the L1 gene, the encoding of the major capsid protein, almost 200 distinct HPV types (genotypes), have been recognized. HPV types 16 and 18 are commonly seen in cancers and considered to be high risk, and other less common types (30, 31, 33, 35, 39, 45, 51 - 53, 56, 58, 59, 66, 68, 73, 82) are somewhat less frequently associated with neoplasms but are also considered high risk (9).
HPV infection does not appear to be involved in ESCC in western countries such as Europe and the United States, but high HPV infection has been seen in ESCC in high-risk countries such as Iran and China (8, 10, 11). A meta-analysis evaluated the possible association of HPV and EC worldwide. The findings indicated that some high-risk HPV genotypes, including 16 and 18 were seen in EC but the results were not consistent (6).
The HPV infection is associated with cancers of the cervix, vagina, vulva, anus, penis, and head and neck. HPV-16 and 18 were seen in all types of cancers. HPV was detected in 23.1% of ESCC in Iran (11).
2. Objectives
In cancerous tissues, the genome of the papillomavirus is integrated into the host chromosome, at which time the viral genes, such as L1 are removed. In studies in which the detection method is based on the L1 gene, there is a potential false-negative result. Therefore, the aim of this study was to evaluate the prevalence of HPV and its association with esophageal cancer, using genotype-specific primers (E6/E7) in West Azerbaijan, Iran.
3. Methods
3.1. Study Population and Specimens
We searched for patients with and without esophageal cancer in the hospital database in Imam Khomeini Hospital, Urmia, West Azerbaijan, north-west of Iran. The patients were referred for treatment due to problems such as pain during swallowing, severe chest pain, inflammation of the esophagus, food reflux from the stomach to the esophagus, cough during meal and weight loss, from which esophageal tissue biopsy samples had been taken, and formalin-fixed and paraffin-embedded (FFPE) and archived in pathology laboratory.
The inclusion criteria in the present study included esophageal tissues without hemorrhage and necrosis. The exclusion criteria included damaged FFPE blocks and tissues without patients’ information.
Overall, 86 samples of FFPE blocks were collected from the archive of the pathology laboratory from 2014 to 2016. In this case-control study, 43 samples suffered from esophageal cancer (cases), and 43 samples were esophageal non-cancerous tissues (controls). Sections with 7 µm to 10 µm in thick were prepared from each block, using a sterile microtome blade and collected in sterile DNase free 1.5 mL microtubes with the precaution of contamination. The microtubes were stored at -20ºC in the freezer until deoxyribonucleic acid (DNA) extraction.
In addition, by using the pathology code, patients’ information was collected, including the case number, age, gender, the grade of malignancy, and place of living (urban or rural). The name of the patients remained unknown and the patient’s personal information was held confidentially. This study was approved by the Ethics Committee of Kurdistan University of Medical Sciences (Ethics code: MUK.REC.1395.213).
3.2. Tissue Deparaffinization
The tissues were deparaffinized by xylene, briefly, 1 mL xylene (Merck, Germany) was poured into each microtube containing 10 tissue sections at room temperature for 15 minutes. The samples were mixed by a vortex every 5 minutes to dissolve the paraffin. Then, the supernatant was separated by centrifugation at 18000 RPM for 1 minute and this step was repeated twice. Then, the samples were washed with 1 mL 100%, 70%, and 50% ethanol solutions for 5 minutes at room temperature. The ethanol supernatant was removed, and the pellet was air-dried for 5 minutes (12).
3.3. DNA Extraction
DNA was extracted from the deparaffinized tissues according to the manufacturer’s instructions (Paraffin-fixed Tissue DNA Extraction Micro Kit, Cat no.: YT9035, Favorgen, Taiwan). DNA concentrations were determined by spectrophotometry. The extracted DNA samples were stored at a temperature of -20ºC until the polymerase chain reaction (PCR) test was carried out.
3.4. Detection of Human Beta-Globin
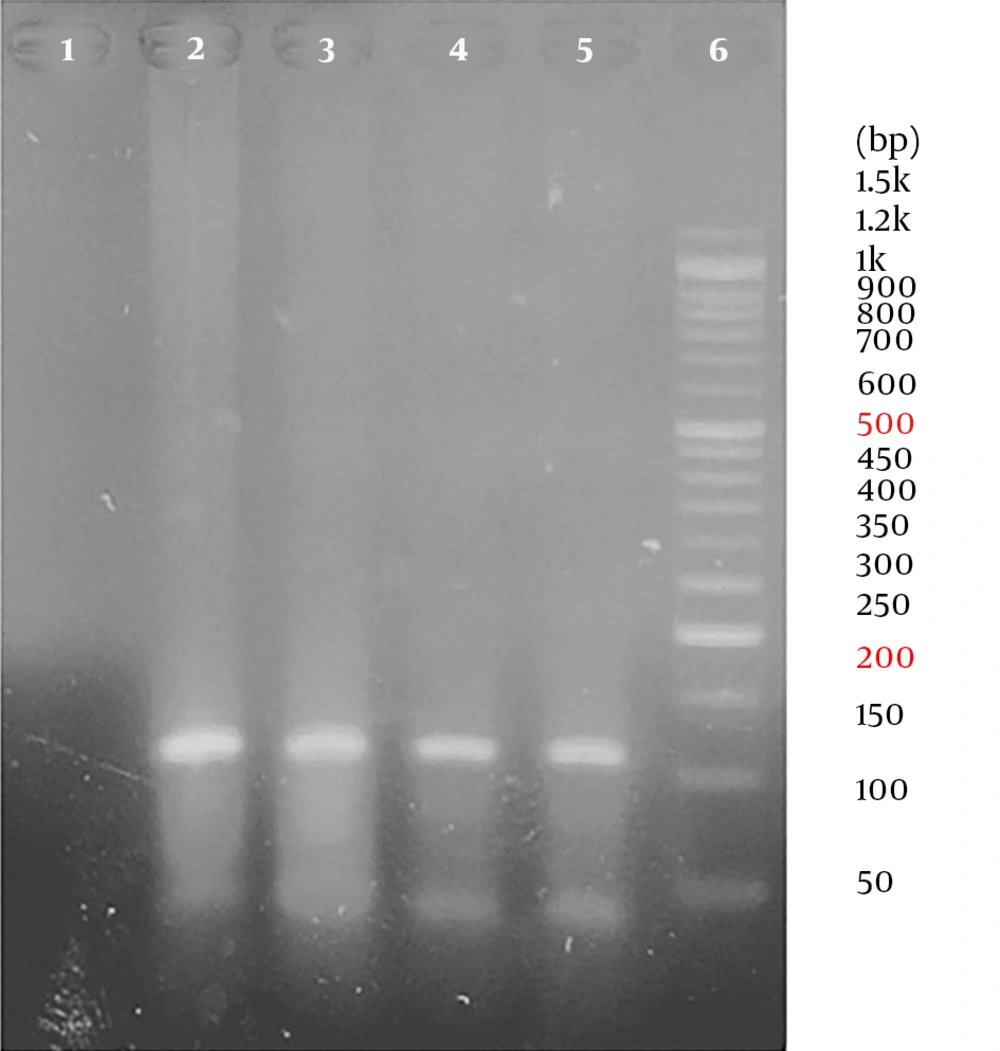
For verifying the integrity of the DNA, PCR was done on all extracted DNA, using primers for the human beta-globin gene (PCO4/PCO3) (Table 1). To reduce the potential DNA contamination, standard laboratory procedures were practiced when handling and processing of specimens in each step. The PCR reaction was done in a total volume of 20 µL mixture containing 10 µL of 2 × master mix (Taq DNA Polymerase Master Mix RED, Ampliqon, Danmark, Cat no.: A160301), 1 µL forward primer (10 µM), 1 µL reverse primer (10 µM) (Table 1), 1 µL DNA template, and 7 µL distilled water. The PCR amplification program for beta-globin included an initial denaturation at 94ºC for 5 minutes, followed by 35 cycles of denaturation at 94ºC for 1 minute, annealing at 55ºC for 1 minute, extension at 72ºC for 30 seconds, and final extension at 72ºC for 10 minutes.
| Primer Name | Primer Sequence | Target | Length of PCR Product, bp | Reference |
|---|---|---|---|---|
| PCO4 | 5’-CAACTTCATCCACGTTCACC-3’ | Beta-globin | 110 | (13) |
| PCO3 | 5’-ACACAACTGTGTTCACTAGC-3’ | |||
| GP5+ | 5’-TTTGTTACTGTGGTAGATACTAC-3’ | L1 | 150 | (14, 15) |
| GP6+ | 5’-GAAAAATAAACTGTAAATCATATTC-3’ | |||
| HPV-16 | 5’-TTATGAGCAATTAAATGACAGCTCAG-3’ | E7 | 215 | (16) |
| 5’-TGAGAACAGATGGGGCACACAAT-3’ | ||||
| HPV-18 | 5’-GACCTTCTATGTCACGAGCAATTA-3’ | E7 | 236 | (16) |
| 5’-TGCACACCACGGACACACAAAG-3’ | ||||
| HPV-31 | 5’-AGCAATTACCCGACAGCTCAGAT-3’ | E7 | 210 | (16) |
| 5’-GTAGAACAGTTGGGGCACACGA-3’ | ||||
| HPV-33 | 5’-ACTGACCTAYACTGCTATGAGCAA-3’ | E6 | 229 | (17) |
| 5’-TGTGCACAGSTAGGGCACACAAT-3’ |
3.5. Detection of Overall and HPV Genotypes
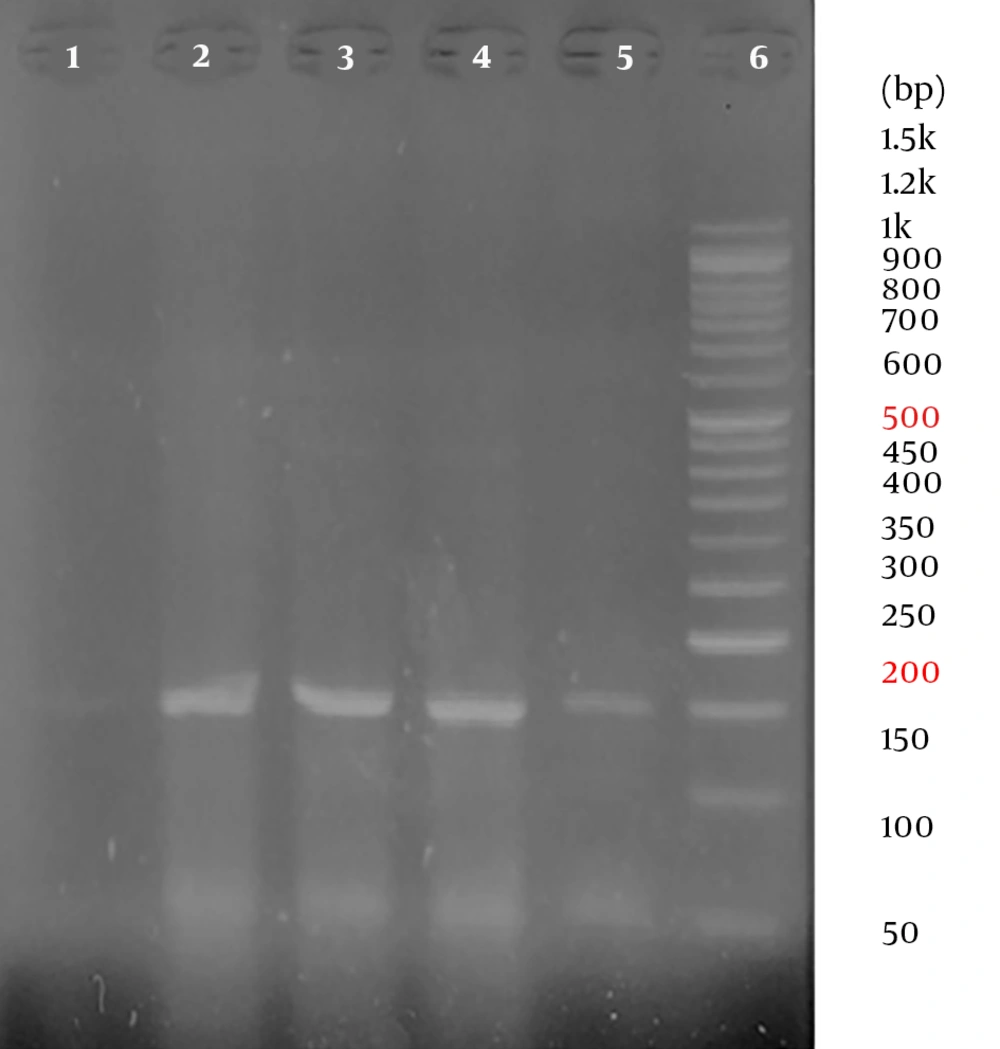
All beta-globin positive samples were tested by PCR to detect overall HPV, using general primers (GP5+/GP6+) designed for the L1 region of the HPV genome (Table 1). The PCR reaction was done in a total volume of 20 µL mixture containing 20 µL of 2 × master mix (Taq DNA Polymerase Master Mix RED, Ampliqon, Denmark, Cat no.: A160301), 1 µL forward primer (10 µM), 1 µL reverse primer (10 µM), 1 µL DNA template, and 7 µL distilled water. The PCR amplification program for HPV detection using general primers included initial denaturation 94ºC for 5 minutes, followed by 35 cycles of denaturation at 94ºC for 1 minute, annealing at 52ºC for 1 minute, extension at 72ºC for 30 seconds, and final extension at 72ºC for 5 minutes.
In addition, the PCR test was done on all samples, using high-risk HPV genotype-specific primers (E6/E7) designed for E6 or E7 genes of the viral genome (Table 1). The PCR reaction was done in a total volume of 20 µL reaction mixture (Ampliqon, Denmark), 1 µL forward primer (10 µM), 1 µL reverse primer (10 µM), 1 µL DNA template, and 7 µL distilled water. The PCR amplification program for HPV included initial denaturation 94ºC for 5 minutes, followed by 35 cycles of denaturation at 94ºC for 1 minute, annealing at (54ºC for HPV-16, 58ºC for HPV-18, 56ºC for HPV-31, and 63ºC for HPV-33) 30 seconds, extension at 72ºC for 30 seconds, and final extension at 72°C for 5 minutes.
DNA was extracted from HeLa cells (National cell bank, Iran Pasteur institute, NCBI Code: C115) and used as a positive control for HPV-18. Positive DNA controls for HPV genotypes 16, 31, and 33 were obtained from Keivan Clinical Virology Lab and Masih Daneshvari Hospital, Tehran. The PCR product separation was done by electrophoresis on 2% agarose gel, stained by SYBR Green, visualized by UV light (Gel transilluminator-BIOView) and photographed.
3.6. Sequencing
In order to confirm the HPV positive results, PCR products (containing DNA band with expected molecular length, Table 1) were sequenced (ABI 3730.1 DNA analyzer, 96-capillary sequencer, Macrogen, Korea). The results of sequencing were compared with the sequences stored in GeneBank, using NCBI nucleotide BLAST software.
3.7. Statistical Analysis
The data were analyzed by SPSS software (version 20). For comparison of qualitative variables, chi-square and Fisher exact tests were used in different groups. P values of less than 0.05 were considered statistically significant.
4. Results
In this case-control study, 43 samples suffered from esophageal cancer and 43 samples were esophageal non-cancerous tissues. The demographic variables, including age, gender, living area of patients in two groups (cases and controls) were equally allocated. The frequency of patients was higher in 40 to 59 and 60 to 79 age groups in both cases and controls. In cases, 36 patients had ESCC and 7 patients had EAC. The patients’ information such as degree of malignancy in the patients is shown in Table 2.
| Variables | Esophageal Cance (Cases), n = 43 | Non-Cancerous Esophageal Tissuesb (Controls), n = 43 | Total, n = 86 | P Value |
|---|---|---|---|---|
| Age groups, y | 64.41 ± 12.22 | 64.81 ± 10.89 | - | 0.785 |
| < 40 | 1 | 1 | 2 | |
| 40 - 59 | 13 | 10 | 23 | |
| 60 - 79 | 25 | 26 | 51 | |
| > 80 | 4 | 6 | 10 | |
| Gender | 0.9 | |||
| Women | 20 | 20 | 40 | |
| Men | 23 | 23 | 46 | |
| Place of living | 0.66 | |||
| City | 23 | 23 | 46 | |
| Village | 20 | 20 | 40 | |
| Type of cancer | ||||
| ESCC | 36 (83.72) | - | 36 | |
| EAC | 7 (16.28) | - | 7 | |
| Degree of malignancy | ||||
| Poorly differentiation | 5 (11.63) | - | - | |
| Moderate differentiation | 31 (72.09) | - | - | |
| Well differentiation | 7 (16.28) | - | - | |
| Overall HPV | 0.078 | |||
| by general primers | 15 | 8 | 23 | |
| HPV genotypes | ||||
| HPV-16 | 3 | 0 | 3 | 0.78 |
| HPV-18 | 10 | 6 | 16 | 0.26 |
| HPV-31 | 1 | 1 | 2 | 0.9 |
| HPV-33 | 1 | 1 | 2 | 0.9 |
Abbreviations: ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma.
aValues are expressed as No. (%) or mean ± SD.
bBiopsies of esophageal non-cancerous tissues from patients with dysphagia, endoscopically normal or margins of carcinomas as control
All samples were positive for the human beta-globin gene. Overall, out of 86 samples, 23 samples were positive for HPV using the general primers. In other words, the overall prevalence of HPV in the population was 26.74%. The frequency of HPV infection was 15 and 8 in patients with esophageal cancers (cases) and in patients with non-cancerous esophageal tissues (controls), respectively. Therefore, the prevalence of HPV was 34.9% and 18.6% of the cases and the controls, respectively. No significant association was observed between HPV and esophageal cancer (P = 0.078) (Table 2). Representative agarose gel electrophoresis of PCR products for the beta-globin gene and HPV detection are shown in Figures 1 and 2.
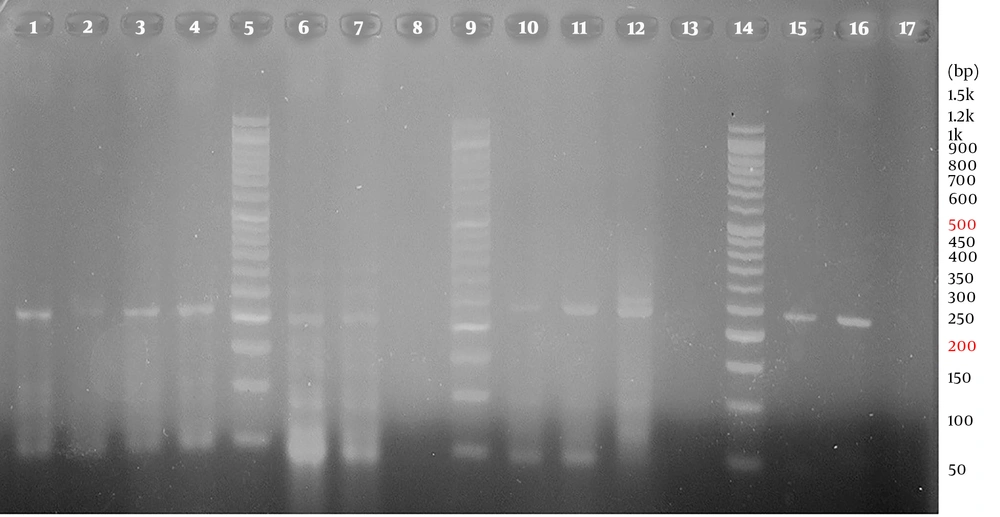
By using HPV type-specific primers, high-risk HPV types were detected in the cases (3 HPV-16, 10 HPV-18, 2 HPV-31, and 1 HPV-33) and the controls (6 HPV-18, 1 HPV-31, and 1 HPV-33). A representative agarose gel electrophoresis of PCR products for the detection of HPV genotypes is shown in Figure 3. The HPV positive samples were verified by sequencing and comparing with the sequences stored in Gene Bank. No significant association was observed between HPV genotypes and esophageal cancer (Table 2).
Representative 2% agarose gel electrophoresis of PCR products for the detection of HPV genotypes by type-specific primers (E6/E7); lane 1, positive control (215 bp), lane 2, negative control, lanes 3 and 4, positive results for HPV-16 (215 bp); lane 6, positive control (210 bp), lane 7, HPV-31 positive result (210 bp), lane 8, negative control; lanes 10, positive control (236 bp), lanes 11 and 12, positive results for HPV-18 (236 bp), lane 13, negative control; lane 15, positive control (229 bp), lane 16, positive results for HPV-33 (229 bp); lanes 5, 9 and 14, DM1100 ExcelBand™ 50 bp DNA ladder (SMOBIO)
In addition, no significant association was observed between HPV and patients’ demographic variables, including age, gender, place of living, type of cancer, and degree of malignancy (Table 2).
5. Discussion
In the present case-control study, we investigated the prevalence and relation of HPV infection with esophageal cancer in West Azerbaijan, Iran. In cases, 36 patients had ESCC and 7 patients had EAC. Overall, out of 86 patients, 23 (26.74%) had HPV infection (15 cases and 8 controls). The prevalence of HPV infection was 34.9% and 18.6% in the cases and controls, respectively. In addition, high-risk HPV types were detected in the cases (3 HPV-16, 10 HPV-18, 2 HPV-31, and 1 HPV-33) and the controls (6 HPV-18, 1 HPV-31, and 1 HPV-33). Although no significant association was observed between HPV infection and esophageal cancer, we found high-risk HPV genotypes in the study population. High-risk HPV genotypes in the cases were more than controls.
A study investigated the association of HPV-16 and 18 infections with the esophageal carcinoma in the world population by a meta-analysis. In 33 randomized studies, the HPV infection rate in the esophageal carcinomas was 46.5%, while the HPV infection rate in the control group was 26.2% (OR = 1.62; 95% CI: 1.33 - 1.98). In China, the odds ratio (OR) was 1.62 (95% CI: 1.26 - 2.07), while in Asia, the OR was 1.63 (95% CI: 1.29 - 2.04), the two areas with the high incidence of the esophageal carcinoma. There were statistical differences in HPV testing methods such as PCR, immunohistochemistry, and in situ hybridization. In the PCR detection group, the OR was 1.61 (95% CI: 1.33 - 1.95). It was concluded that HPV infection is associated with the incidence of esophageal carcinoma (6). In comparison to the prevalence of HPV in the world population, the prevalence of HPV in our case and control groups was low.
In a study conducted in Turkey, the presence of HPV infection was investigated by real-time PCR in 52 patients with esophageal cancer. Out of 52 samples, 5 (9.6%) were positive for HPV, including 3 of 33 ESCC and 2 of 19 EAC cases. Genotypes of 4 HPV-positive cases were detected, of which 3 were HPV-39 and 1 was HPV-16. It was concluded that HPV infection, particularly high-risk genotypes, may have a role in esophageal carcinogenesis (18). In comparison to the prevalence of HPV in Turkey, the north-west neighbor of Iran, the prevalence of HPV was higher in the study population (West Azerbaijan, north-west of Iran).
A study was conducted on 96 esophageal samples (51 ESCC and 45 non-cancerous) in Mazandaran, north of Iran, with a high incidence of ESCC. Real-time PCR was conducted to detect HPV using consensus L1 primers (MY09/MY11). AmpliSense HPV real-time fluorescence detection kit was used for HPV genotyping. HPV DNA was detected in 16 (31.4%) out of 51 ESCC (cases) and 20 (44.4%) out of the 45 non-cancerous samples (controls). In addition, Merkel cell polyomavirus DNA was detected in 23 (45.1%) out of the 51 ESCC cases and 16 (35.6%) out of 45 non-cancerous samples. There was no significant difference between cancerous and non-cancerous samples according to HPV and Merkel cell polyomavirus (3). Therefore, other oncogenic viruses may have a role in esophageal cancer, but we did not try to evaluate other oncogenic viruses in the study population.
In a study, the role of HPV infection in ESCC cases was evaluated in the Kurdish population in Kurdistan and Kermanshah Provinces, west of Iran; 103 ESCC samples were investigated from 2007 to 2013. HPV and its genotypes were detected by PCR using consensus primers for the HPV L1 gene and the INNO-LiPA genotyping kit. HPV DNA was positive in 11/103 (10.7%) of ESCC cases. Out of 11 HPV positive samples, 5 were HPV-18 and 6 were HPV-16. The co-infection of HPV-6 and HPV-18 was found in 2 samples. There was no statistically significant association between HPV and clinical/pathologic findings. The authors concluded that HPV could be a risk factor for ESCC among low-risk ESCC regions in two provinces in the west of Iran (8). In comparison to the prevalence of HPV in the western provinces near the area studied in this research, the prevalence of HPV in our patients was higher.
The frequency of HPV infection was determined in 80 biopsy samples of non-cancerous esophageal lesions after an upper endoscopy in Babol, Mazandaran, north of Iran. Out of 80 samples, 29 (36.3%) were positive for HPV using the HPV L1 primers (MY09/MY11). Then, genotypes were detected in HPV positive samples by real-time PCR. Genotypes of 14 HPV positive samples were detected. HPV-11 was the dominant genotype, but any high-risk genotypes (HPV-16 and HPV-18) were not found. The authors concluded that they did not find any high oncogenic HPV-16 and 18 genotypes (19).
In a study, the prevalence of HPV was determined in ESCC cases in Iranian patients from 2007 to 2009 in Cancer Institute, Tehran; 30 ESCC cases were randomly selected. In addition, 30 samples of gastroesophagectomy with an involved esophageal margin and normal esophageal tissues were selected as controls. DNA was extracted from all samples by the same method with the strict control of contamination to prevent false-positive results. PCR test was conducted, using GP5+/GP6+ primers targeted to the sequence of the HPV L1 gene. HPV was negative in both groups (case and control). The authors concluded that HPV had no role in ESCC in their patients (20).
The prevalence of HPV was determined in ESCC cases in Shiraz, south of Iran. DNA was extracted from FFPE tissues from 92 ESCC cases over 20 years (1982 - 2002). PCR test was done for the detection of HPV genotypes 16 and 18, using consensus L1 primers. HPV-18 positive biopsies from uterine exocervix were used as a positive control, and DNA integrity was verified by beta-globin and cytotoxic T cell antigen 4 sequences. No HPV was detected and there was no association between HPV infection and the development of ESCC (21).
In a study, the frequency of HPV infection and its genotypes were determined in ESCC cases in Iran; 93 FFPE samples from ESCC cases were selected from 1991 to 2005 in the Cancer Institute, Tehran. All samples were evaluated by the PCR test and SPF10 primers for HPV L1 sequences. HPV was positive in 8 (8.6%) of 93 ESCC cases. HPV genotypes were detected in 5 out of 8 HPV-positive samples, using the INNO-LiPA genotyping kit. HPV-16 and HPV-6 were detected in 3 samples. HPV-18 was positive in 1 sample, and 2 samples were co-infected by 2 HPV genotypes. In 3 samples, the genotypes HPV could not be detected. No significant difference was observed between HPV-positive and HPV-negative cases according to clinical and pathologic findings (22).
In a case-control study, 40 ESCC cases and 40 nonmalignant specimens (controls) were collected in Sari, Mazandaran, north of Iran, from 2001 to 2008. DNA was extracted from the specimens and was analyzed for HPV DNA with Amplisense kit (Russia); 15 (37.5%) of ESCC cases and 5 (12.5%) of controls were positive for HPV. The dominant genotypes in the ESCC cases and controls were HPV-16 and HPV-45. No significant association was seen among HPV genotypes and the patients’ age, sex, tumor stage, and grade. The authors concluded that HPV might be an important risk factor for ESCC in the north region of Iran (23).
In a study, HPV infection was evaluated in Guilan, north of Iran, with a high incidence of ESCC. Three kinds of primers were used, including general primers (GP5+/GP6+) to detect the overall prevalence of HPV, genotype-specific primers to detect mild oncogenic genotypes (HPV-31, 33, 35, 39, 41, 51, 52) and E6/E7 primers to detect high-risk oncogenic genotypes (HPV-16 and 18) (Isogen kit, Russia). Out of 45 ESCC cases, 17 (37.7%) samples were positive for HPV, 4 (8.8%) samples were positive for mild oncogenic genotypes, 2 (4.4%) samples were positive for high-risk genotypes (HPV-16 and18), and 22 samples were HPV negative. It was concluded that the results were compatible with the results of HPV studies conducted in Guilan, a high-risk area for ESCC (24).
In a study, the role of HPV on ESCC was evaluated among 140 ESCC cases in Tehran. PCR was done, using general primers (GP5+/GP6+) for the L1 gene of HPV. The PCR products were sequenced to identify the genotypes of HPV. Out of 140 patients, 50.7% were female and 49.3% were male with the age range of 20 to 81 years. HPV was positive in 33 (23.6%) ESCC cases and 12 (8.6%) controls (non-involved tumor margins). From HPV positive cases, 21.7% were male and 25.3% were female. There was no association between HPV and patients' age and gender. The prevalence of HPV genotypes in ESCC cases was as follows: HPV-16 (60.6%), HPV-18 (30.3%), HPV-33 (6.1%), and HPV-31 (3%). Only HPV-16 was detected in the controls (25).
The prevalence of HPV differs among geographical regions and according to the detection methods (6). Based on a meta-analysis of cervical cancer, the rank of HPV detection primers from most to least sensitive order were as follows: SPF10, GP5+/GP6+, L1C1/2, MY09/MY11, PU1M/2R, GP5/GP6, L1, E6, E7, and HPV-16 and 18 specifics (26).
In the present study, two common and standard molecular methods for the detection of HPV and its genotypes were used and, then, confirmed by sequencing. The results were consistent with the results of some previous studies that evaluated the prevalence of HPV in ESCC cases in high-risk or low-risk areas of Iran. The findings of the present study provide further evidence to support the probable role of HPV infection in esophageal cancer.
Specimen type may affect the result of HPV detection. Specimens in all previous studies included biopsies from esophageal tissues, such as FFPE tissue blocks. The specimens in our study were also FFPE tissue blocks. Therefore, the differences in the results in our study and previous studies could not be due to a difference in specimen types.
In the present study, no significant association was observed between HPV and esophageal cancer (P = 0.078). However, high-risk HPVs in the esophageal cancer group (cases) were more than non-cancerous esophageal tissues (controls). Regarding the P value, if the study was done with a larger sample size, the association would probably be significant. Perhaps, other factors have a tumorogenesis role in esophageal cancer in the study population. We did not try to evaluate other factors such as other oncogenic viruses. However, because of the probable detection failure and population variations, it is better to confirm the findings of the present study by different detection methods and in other large populations.
5.1. Conclusions
Although in the present study no significant association was observed between HPV and esophageal cancer, high-risk HPVs were found in the study population, and HPVs in esophageal cancer group (cases) were more than non-cancerous esophageal tissues (controls). With respect to the defined oncogenic role of HPV in some cancers such as cervical cancers, the causality of HPV in esophageal cancer remains elusive; so, more studies with larger sample sizes, using different laboratory methods and in other populations is necessary.