1. Background
Breast cancer, being the most frequent form of cancer among women, represents a serious problem in modern society. As defined by the European Society of Mastology (EUSOMA) and then by the European guidelines, documents, and scientific papers (1-11) breast cancer care requires a multidisciplinary and well- structured approach. Experts in different fields need to join their competencies and work in specialized breast cancer clinics (breast units) where patients receive high-quality personalized care. While most of the past scientific papers focused on the importance of process quality indicators of breast cancer care (e.g. the minimal/optimal composition of the expert team, compliance to care protocols, re-hospitalization, surgical site infections, etc.) (12, 13), only few studies have provided a quantitative approach on patient’s perspective (14-19). It is very useful to collect the patient’s opinion when the performance of breast unit professionals are evaluated. It has been shown that patient’s feedback improves physicians’ team performance and care quality. Furthermore, at the organizational level patient’s satisfaction may provide useful information about the ability of hospital to provide good service as a part of the patient’s experience (20-25).
According to the authors’ knowledge, two questionnaires have been developed with the aim to evaluate patient’s perspective on the breast cancer care: the quality of care through the patients’ eyes breast cancer (QUOTE-BC) questionnaire (17); and the consumer quality index (CQ-index) breast care instrument (14).
For the purpose of the current study, we chose the QUOTE-BC questionnaire so that we only assess the aspects of hospital breast cancer care, instead of the CQ-index items which are usually focused on assessing other health care sectors (e.g. relation with the general practitioner).
The QUOTE-BC is a questionnaire which was developed by the Netherland Institute of Public Health. It is specifically designed to provide data on how breast cancer patients experience healthcare services, to explore issues related to patients’ needs and expectations and to produce useful data for quality assessment in breast cancer care. Several examples of translation and cultural adaptation of instruments using the QUOTE methodology have been already reported for different health conditions in different languages (26-31).
Based on the best of the authors’ knowledge, none of the existing instruments to evaluate the patients’ experience have yet been translated, adapted, and validated for the Italian population.
Therefore, the aim of this methodological study was to validate a cultural and organizational adaptation of the QUOTE-BC for its use in Italian breast units.
The questionnaire comprised 33 items covering the most important aspects of breast cancer care, from diagnostic tests to adjuvant treatment, assessing both the quality of care and the performance provided by healthcare professionals. Patients were asked to rate perceived performance of the healthcare professionals/services. Four-point Likert-type scales (never; sometimes; usually; always) were used for most questions, while dichotomous answering categories (yes/no) were applied for the rest of the items. The subscales referred to: (1) patient education regarding postoperative treatment (6 Likert-type items); (2) services by the breast nurse (5 Likert-type items); (3) services by the surgeon (6 Likert-type items); (4) patient education regarding activities at home (3 dichotomous items); (5) patient education regarding aspects related to preoperative treatment (4 dichotomous items). The questionnaire also comprised a set of 9 miscellaneous items that were not grouped into a specific scale. The resulting set of items was easy to complete and enabled anonymous responses. The instrument combines experience and needs of breast cancer patients with outcome measures, offering specific information about the quality of specific aspects of breast cancer care, which can become targets for quality improvement actions.
2. Methods
2.1. Study Overview
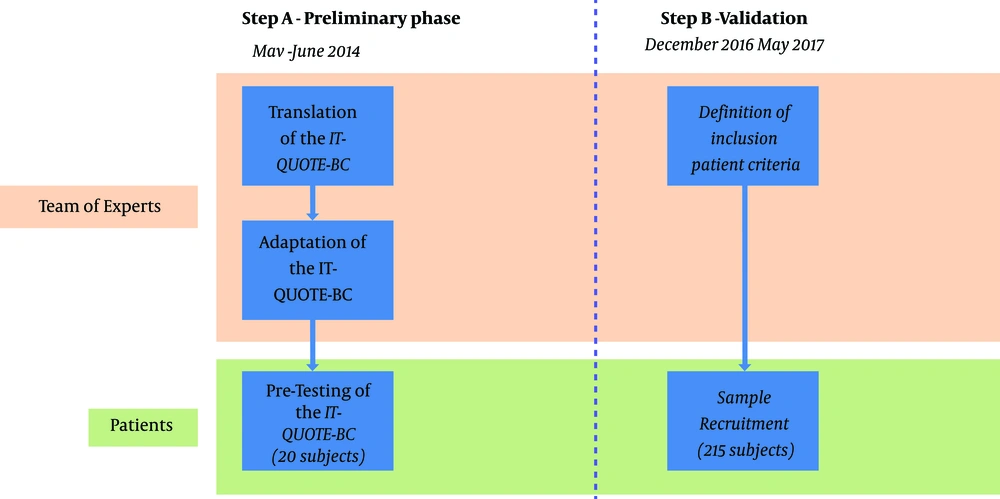
Due to the methodological nature of this study, the research was divided into two separate steps (Figure 1).
•From May to June 2014, a preliminary phase was carried out involving translation, adaptation, and pre-test of the questionnaire in Italy on a small sample of patients in breast unit, resulting in the assessment of the Italian version of the QUOTE-BC (IT-QUOTE-BC) adapted to the Italian population;
•From December 2016 to May 2017, data collection activity was performed by administering the previously developed IT-QUOTE-BC to a large group of patients to further validate the questionnaire by assessing its factorial structure, internal consistency, and construct validity.
2.1.1. Step A: Translation, Adaptation, and Pre-Testing of the IT-QUOTE-BC
The translation process was carried out by following established good process; first, the original English tool was initially distributed among a team of experts (1 breast surgeon, 2 epidemiologists, 1 public health doctor, and 1 general surgery resident). Translation from English into Italian was carried out by each member, resulting in an initial collection of different translations which were later merged into a single one by the whole team. In order to check the translation reliability, the resulting version was translated back into English and evaluated by the team. Finally, the team filled out the questionnaire to detect any possible ambiguities and misinterpretations (32). To further pre-test the adapted version for comprehensibility, the questionnaire was administered to a convenience sample of 20 breast cancer patients who were also interviewed to check possible misinterpretations.
In line with previously reported adaptations of similar QUOTE questionnaires (26-28, 31), a visual analogue scale (VAS) was added at the end of the questionnaire to detect the patients’ satisfaction on the quality of breast unit. The patients had to mark a 10 cm horizontal line that varies from 0 (totally dissatisfied) to 10 (very satisfied) (33). The IT-QUOTE-BC is available upon request from the corresponding author.
2.1.2. Step B: IT-QUOTE-BC Validation
To validate the IT-QUOTE-BC we conducted a field study by recruiting patients in breast units located in north-eastern regions of Italy (Autonomous Province of Trento, Friuli Venezia Giulia, and Veneto). In order to collect the most comprehensive sample possible, all patients accessing the breast units from December 2016 to May 2017 were initially screened for eligibility. Patients were eligible if: (a) scheduled for surgery for any type of breast cancer, (b) aged 18 years or older, (c) mentally competent as judged by doctors, and (d) hospitalized in the breast unit for at least 2 days. All study participants were female. Patients with pre-neoplastic lesions and who underwent on a day-surgery setting were excluded from the study due to short stay at the hospital. Surgeons of breast units performed recruitment and invited the patients to participate in the hospital admission study. After briefing about the study, patients were asked to sign informed consent to be included as study participants. During the first medical examination after surgery, participants received a paper sheet of the IT-QUOTE-BC questionnaire to fill it at home to improve acceptability and comfort while answering. Demographic information (date of birth, residence, educational level, and occupational condition) was also collected. The questionnaire was then returned in a closed envelope during the following outpatient visit and scheduled within 10 - 15 days.
2.2. Statistical Analysis
A descriptive analysis of the sample data collected in the second step was performed. Frequencies and percentages were calculated for categorical variables, while the measures of central tendency and dispersion were calculated for continuous ones. A confirmatory factor analysis (CFA) was then conducted by confirming the structural validity and the correct assignment of each item to the respective dimension, like the original version of the QUOTE questionnaire. Cronbach alpha was calculated to test the internal consistency for each factor of the IT-QUOTE-BC: 0.70 or higher values were considered optimal (34). As the questionnaire was supposed to measure the patient’s perspective during the care process, we decided to test its construct validity by comparing IT-QUOTE-BC results with a summary measure of patients’ satisfaction using a VAS “from 1 to 10, how much do you feel satisfied with the received treatment?”
All Likert-type variables were dichotomized (never, sometimes = negative answer group; usually, always = positive answer group) and compared using the Wilcoxon-Mann-Whitney test to find any statistical difference in VAS values between patients who gave positive answers and patients who gave negative answers. The significance level for all tests was set to α = 0.05.
All the analyses were performed with SAS 9.3 software for Windows (SAS Institute Inc., Cary, North Carolina, USA).
3. Results
The following changes were required in order to provide a cultural adaptation of the original QUOTE-BC instrument to further adapt it to the Italian organizational standards:
1. There was a minor revision in the item of original questionnaire in order to allow immunohistochemistry characterization to be performed on local standards of care. (“The results of a biopsy were communicated to me within three (working) days” was modified in “The results of a biopsy were communicated to me within seven (working) days”);
2. Since complete diagnostic results, including double-blinded read mammography performed by two expert breast radiologists, require more than 24 hours to meet local standards, the following item of the original questionnaire was eliminated: “Within 24h after diagnostic tests (mammography, breast ultrasound and a possible fine-needle biopsy)”;
3. To further explore standard quality of Italian breast units requirements, the original item on surgery timing “I was operated within 2 weeks from being diagnosed as having breast cancer” was modified into “How long did you wait for surgery after diagnosis?”, with three possible answers: within four weeks, between 4 and 6 weeks, and more than 6 weeks. The surgeons filled out this question before giving the questionnaire to the participants.
A total number of 215 breast cancer patients agreed to take part in the study and 98.6% (212) of them returned the questionnaire.
Table 1 demonstrates the socio-demographic characteristics of the participants, including information about the time between the first visit and surgery. The average age of the women who participated in the study was 59.8 years old (from 22 to 86), with a mid-low educational level (40%), and most of them were not working at the time of interview. The surgery was carried out within 6 weeks from the diagnosis for most of the participants (75%). The VAS descriptive analysis showed that 97% of the respondents had expressed a positive opinion about the quality of the care experience (median = 10).
| Variables | Values |
|---|---|
| Age | |
| Mean ± SD | 59.8 ± 13.0 |
| Median | 61.5 |
| Min - max | 22 - 86 |
| Educational attainment, No. (%) | |
| Low | 49 (23.0) |
| Middle | 36 (17.0) |
| High | 16 (7.6) |
| NR | 111 (52.4) |
| Working status, No. (%) | |
| Working | 79 (37.3) |
| Not working | 114 (53.8) |
| NR | 19 (8.9) |
| Time between breast visit and surgery, No. (%) | |
| < 4 weeks | 80 (37.7) |
| Between 4 and 6 weeks | 79 (37.3) |
| > 6 weeks | 32 (15.1) |
| NR | 21 (9.9) |
| VAS (n = 206) | |
| Mean ± SD | 9.2 ± 1.07 |
| Median | 10 |
| Min - max | 5 - 10 |
Abbreviation: VAS, a visual analogue scale
Applying CFA, we confirmed the five-factor structure of the original questionnaire which explains the 80.2% of the total variance, and the root mean square error of approximation (RMSEA) was good (= 0.01)
Results from internal consistency analysis of the sub-scales are presented in Table 2. Except for sub-scale 2, “services by the breast nurse” (alpha = 0.62), all the other sub-scales presented a good level of internal consistency, with an alpha value between 0.69 and 0.84. Although some items had a low correlation with the belonging factor (e.g. item 3.6 correlation is 0.25), the results indicated that the questionnaire presents an acceptable overall internal consistency.
| Factors and the Related Items | Correlation with the Factors | Alpha |
|---|---|---|
| Patient’s education regarding postoperative treatment | 0.84 | |
| 1.1. Health professionals informed me well about the possible side effects of the treatment | 0.56 | |
| 1.2. Health professionals informed me well about a possible drain | 0.59 | |
| 1.3. Health professionals informed me well about a possible prosthesis | 0.41 | |
| 1.4. Health professionals informed me well about possible side effects of the treatment | 0.74 | |
| 1.5. Health professionals concerned with adjuvant treatment informed me clearly about the start of adjuvant treatment(s) | 0.75 | |
| 1.6. Health professionals concerned with adjuvant treatment informed me clearly about the consequences of adjuvant treatment (e.g., tiredness, boldness, swollen arm) | 0.67 | |
| Services by the breast nurse | 0.62 | |
| 2.1. The breast nurse listened to me attentively | 0.34 | |
| 2.2. The breast nurse treated me as an equal | 0.28 | |
| 2.3. The breast nurse took me seriously | 0.46 | |
| 2.4. The breast nurse spent enough time on my consultation | 0.50 | |
| 2.5. The breast nurse explained things to me in understandable language | 0.29 | |
| Services by the surgeon | 0.78 | |
| 3.1. The surgeon listened to me attentively | 0.49 | |
| 3.2. The surgeon treated me with respect | 0.64 | |
| 3.3. The surgeon took me seriously | 0.67 | |
| 3.4. The surgeon spent enough time on my consultation | 0.63 | |
| 3.5. The surgeon explained things to me in understandable language | 0.48 | |
| 3.6. The surgeon was informed about my life before the consultation started | 0.25 | |
| Patient education regarding activities at home | 0.69 | |
| 4.1. Health professionals informed me well about wound care in the home situation | 0.47 | |
| 4.2. Health professionals informed me clearly about what I should and should not do after surgery | 0.52 | |
| 4.3. Health professionals informed me well about exercises for the period after surgery | 0.51 | |
| Patient education regarding aspects related to preoperative treatment | 0.79 | |
| 5.1. Health professionals informed me well about survival rates that were known about my type of breast cancer | 0.43 | |
| 5.2. Health professionals informed me well about different treatment options | 0.67 | |
| 5.3. Health professionals informed me clearly about risks of different treatment options | 0.72 | |
| 5.4. Health professionals informed me/ provided me with good information about what the chosen treatment consisted of | 0.58 |
The results of the Wilcoxon-Mann-Whitney test which compares the VAS measure of patients’ satisfaction between the two groups of patients (positive and negative answers) are presented in Table 3. Most of the items showed that the VAS significantly differed between two groups of patients, with only four exceptions (items 2.5, 3.5, 3.6 and 6.2). Overall, these results showed that IT-QUOTE-BC items are significantly related to patients’ satisfaction.
| IT-QUOTE-BC Items | Wilcoxon Mann-Whitney (P Value) |
|---|---|
| Patient education regarding postoperative treatment | |
| 1.1. Health professionals informed me well about possible side effects of the treatment | < 0.001 |
| 1.2. Health professionals informed me well about a possible drain | < 0.001 |
| 1.3. Health professionals informed me well about a possible prosthesis | < 0.001 |
| 1.4. Health professionals informed me well about possible side effects of the treatment | < 0.001 |
| 1.5. Health professionals concerned with adjuvant treatment informed me clearly about the start of adjuvant treatment (s) | < 0.001 |
| 1.6. Health professionals concerned with adjuvant treatment informed me clearly about the consequences of adjuvant treatment (e.g., tiredness, boldness, swollen arm) | < 0.001 |
| Services by the breast nurse | |
| 2.1. The breast nurse listened to me attentively | < 0.001 |
| 2.2. The breast nurse treated me as an equal | < 0.001 |
| 2.3. The breast nurse took me seriously | 0.019 |
| 2.4. The breast nurse spent enough time on my consultation | < 0.001 |
| 2.5. The breast nurse explained things to me in understandable language | 0.173a |
| Services by the surgeon | |
| 3.1. The surgeon listened to me attentively | 0.010 |
| 3.2. The surgeon treated me with respect | 0.065a |
| 3.3. The surgeon took me seriously | 0.014 |
| 3.4. The surgeon spent enough time on my consultation | 0.004 |
| 3.5. The surgeon explained things to me in understandable language | 0.059a |
| 3.6. The surgeon was informed about my file before the consultation started | 0.153a |
| Patient education regarding activities at home | |
| 4.1. Health professionals informed me well about wound care in the home situation | 0.0019 |
| 4.2. Health professionals informed me clearly about what I should and should not do after surgery | 0.0004 |
| 4.3. Health professionals informed me well about exercises for the period after surgery | 0.0002 |
| Patient education regarding aspects related to preoperative treatment | |
| 5.1. Health professionals informed me well about survival rates that were known about my type of breast cancer | 0.007 |
| 5.2. Health professionals informed me well about different treatment options | 0.009 |
| 5.3. Health professionals informed me clearly about risks of different treatment options | < 0.001 |
| 5.4. Health professionals informed me/ provided me with good information about what the chosen treatment consisted of | 0.005 |
| Other items | |
| 6.1. Health professionals took care that the various caregivers coordinated patient education | < 0.001 |
| 6.2. Health professionals had results available when I had an appointment with (one of) them for that reason | 0.063a |
| 6.4. Health professionals ensured that all diagnostic tests took place on the same day | < 0.001 |
| 6.5. The results of a biopsy were communicated to me within ten (working) days | 0.003 |
| 6.6. Health professionals concerned with adjuvant treatment paid me personal attention | < 0.001 |
| 6.7. Health professionals concerned with adjuvant treatment were helpful | < 0.001 |
| 6.8. Health professionals concerned with adjuvant treatment kept appointments punctually | < 0.001 |
aDifference in VAS between positive and negative answer groups not statistically significant.
4. Discussion
In this study we developed an Italian cultural and organizational adaptation of the QUOTE-BC questionnaire and tested its validity for its use in Italian breast units. Since other published adaptations of questionnaires using QUOTE methodology was targeted at different health conditions (26-31), therefore, to compare the result of this research with other related studies we found only one study which carried out by de Kok et al. (17). Validity and comprehensibility of IT-QUOTE-BC were ensured by carefully translating and pre-testing the questionnaire. Overall, we found 3 items that should be changed compared to the original QUOTE-BC instrument.
Regarding the questionnaire structure, factorial analysis confirmed the original 5 sub-scales structure, as described by de Kok et al. (17). Internal consistency of the sub-scales was also assessed using Cronbach alphas and can be considered sufficient for all sub-scales with the exception of sub-scale 2 “Services by the breast nurse”. This result, is not in line with the original study (all alphas equal or above 0.70), and shows the need to further adapt the Italian version to better reflect the cultural aspect of nurse care.
The correlation of the IT-QUOTE-BC items with the VAS was statistically significant for most of the items, suggesting that the questionnaire is indeed related to patients’ satisfaction demonstrating its overall construct validity.
The compliance of participants in the study was 98.6% which is much higher than the previous in the original study by de Kok et al. (47%) (17). We believe this result was due to the different methods of questionnaires distribution and collection: instead of sending out the questionnaire by mail, we delivered and collected it in person during the first medical examination after surgery. Moreover, the number of missing answers in the questionnaires was also very low, which indicates that the surgeons participating in the data collection presented the questionnaire clearly.
In the most of the items of questionnaire structure, the level of patients’ satisfaction showed a statistically significant difference between the two groups of patients (the positive answer group and the negative answer group); the only exception was found in some items regarding the surgeon. In three items regarding the services offered by the surgeon (3.2, 3.5 and 3.6) the VAS measurement in each group of answers is the same.
Both groups agreed on claiming that the surgeons treat the patients with respect, use clear language to explain the situation, and demonstrate their knowledge of the patient’s clinical history at the time of the medical examination.
Especially, the items 3.2 and 3.3 were very similar, as the P value was significant; therefore, in the future, to avoid a possible misunderstanding by the patients, we might consider to unify the two items, as already indicated in the nursing section (2.2 and 2.3), in order to avoid a possible misunderstanding by the patients. The same can be said for the patient-nurse relationship.
However, considering the wide gap between the two groups of answers, it cannot be excluded that the results obtained are due to mere chance. Therefore, the sample needs to be extended to better understand the level of patients’ satisfaction regarding the services offered by the ward and the healthcare personnel.
4.1. Conclusions
While there are many studies that focused on quality indicators of breast cancer care only a few studies had attempted to determine patient’s perspectives on quality of care. A greater understanding of the patients’ perspective is important to provide and improve services and healthcare organizations. As a result of this study, we provided an adapted Italian translation of QUOTE Breast Cancer questionnaire for the first time, in order to assess the quality of care for female breast cancer patients. By introducing the patient point of view, the IT-QUOTE-BC questionnaire can offer valuable support for those who monitor the clinical and the organizational decisions in the Italian breast units routine. The use of the IT-QUOTE-BC questionnaire in the same breast unit will allow to verify whether the organizational changes lead to an improvement in the quality perceived by the patients, besides the usual performance measurement to support managerial and clinical decisions (35). Nonetheless, further refining and testing of the IT-QUOTE-BC still need to be performed to be able fully to validate a reliable instrument. For this purpose, a larger multicentric study is needed.
4.2. Limitations
This study had two main limitations worth considering.
First, we used an opportunity sampling as a collection strategy which limited the generalization of the results. It was obtained as a result of collaboration among the surgeons of the breast units interested in assessing the quality of breast care in their surgical wards.
Secondly, the concentration of the patients’ sample in north-east Italy cannot be considered representative of the whole Italian breast cancer patients. Future studies using the IT-QUOTE-BC should collect data from different Italian regions and compare the results with more objective clinical quality of care metrics (e.g. re-hospitalization, patient infection, discharge time, etc).