1. Introduction
Plasma cell neoplasms are discrete, solitary masses of lymphoid neoplastic proliferation of B cells (1). Solitary bone plasmacytoma (SBP) and extramedullary plasmacytoma (EMP) are the unifocal and monoclonal proliferations of plasma cells, while, multiple myeloma (MM) is the multicentric and disseminated type of the disorder (1-5). The incidence of plasmacytoma tumors is about 2.6 to 3.3 per 100,000 of the population with stronger predilection in blacks (6). The SBP accounts for 3% to 10% of all plasma cell tumors (1, 5). It usually occurs in male patients during the sixth to seventh decade of their life (7-9). A well-defined uni or multilocular lesion without periosteal reaction (2) or a protruding mass with the cortical expansion is normally seen in radiographic images of SBP (10). Pain is the most common sign which is sometimes associated with pathological fractures (11). Due to variable biological behavior, these tumors can have periods of clinical latency to rapid growth and progression from the localized forms (SBP and EMP) to disseminated form (MM) and many authors have considered SBP as an early manifestation of MM with the progression rate of 65% to 100% in 15 years (12). Here we present a patient with SBP and discuss the diagnosis, treatment plan, and the follow-up data with a discussion on the literature.
2. Case Presentation
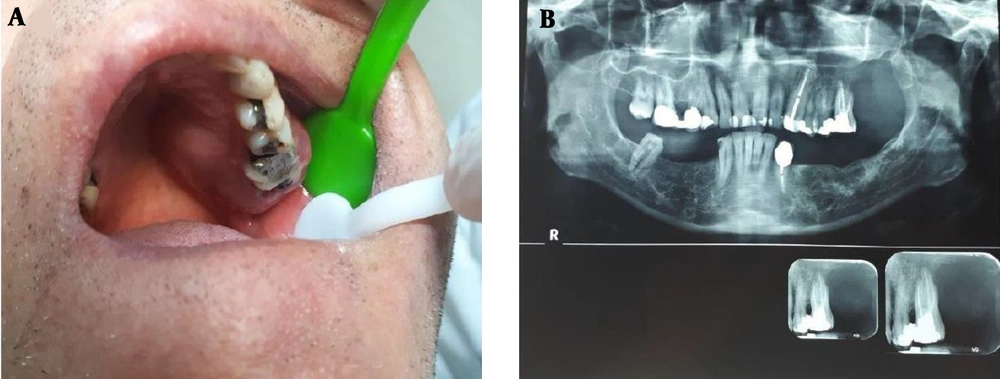
A 59-year old male patient was referred to the school of dentistry with chief complaint of a painful and slowly growing mass of the upper jaw. It was reported that the mass had been enlarged following second molar extraction within four months. He had no significant clinical background and there were no signs of paresthesia, numbness, or other neurological symptoms in the affected area. Intraoral clinical examinations revealed a 5 cm mass with well-defined borders and an intact surface located in the posterior part of left maxillary bone extending from the midline through the depth of the vestibule (Figure 1). Cone beam computed tomography (CBCT) confirmed the existence of a poorly defined lesion in left maxilla, extending from distal area of left upper canine toward pterygoid plates, with destruction of alveolar bone and loss of lamina dura at periapical area of involved teeth, loss of palatal cortical plates, and interrupted border of lateral wall of left maxillary sinus. The incisional biopsy was taken from the lesion without any abnormal bleeding during the procedure. The microscopic evaluation and immunohistochemical studies showed a plasma cell rich tumor, positive for CD138 and Lambda light chain and negative for kappa, CD45, and CD56 which confirmed the diffuse and monoclonal population of cells, indicative for the diagnosis of plasma cell tumor (Figure 2). In order to differentiate between a solitary plasmacytoma and the disseminated disease of multiple myeloma, a systemic workup was done. The FDG-PET/CT scan showed no other involvement except this mild metabolically active tumoral mass in the left maxillary sinus with extension medially to the left nasal cavity and inferiorly to the maxillary bone. There was also no evidence of bone marrow involvement in the aspiration. Serum protein electrophoresis revealed normal total protein (7.5 g/dL) and gamma globulin (14.9%). Ig levels (IgG 1436 mg/dL, IgA 224 mf/dL, IgM 44 mg/dL), hemoglobin, platelets level, leukocytes, serum electrolytes, renal function tests, serum calcium, erythrocyte sedimentation rates, serum beta 2 microglobulin level (2.14 mg/dL) were also within the normal limits. Blood and urine immunofixation studies for M-protein or Bence Jones protein were also negative. According to these findings, the diagnosis of solitary plasmacytoma of the maxillary bone was confirmed for this patient. Due to the poorly-defined lesion and inaccessible surgical margins, the oncologists decided the chemo-radiation treatment instead of surgical resection of the tumor. The patient underwent four sessions of chemotherapy with Bortezomib plus Dexamethasone (BD therapy) and also 10 sessions of radiation with planned dosing of 40 - 50 Gy. The tumor completely regressed after the treatment and the laboratory results remained within the normal limits except a slightly decreased level of IgM (33 mg/dL). The patient has been continued to follow up on an outpatient basis for 18 months and there are no signs of recurrence or progression of the disease to date.
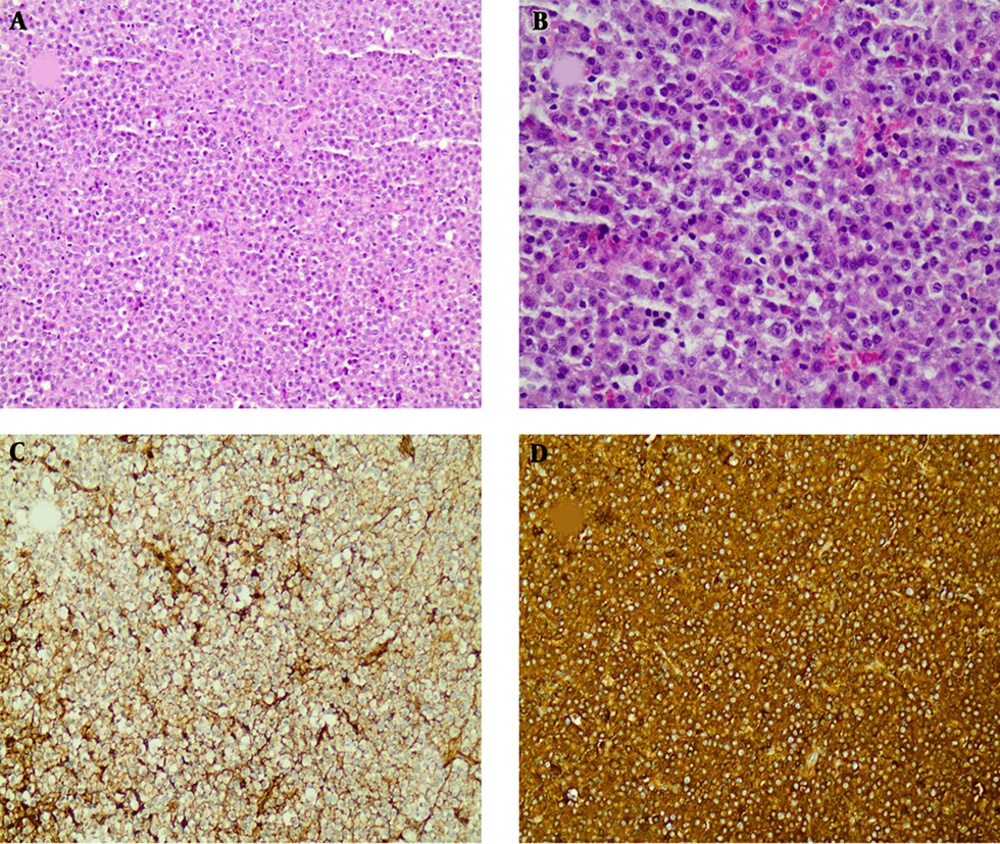
Histopathologic findings showed sheets of diffuse and monotonous neoplastic plasma cells with eccentric nuclei and stippled nuclear chromatin (A-200x and B-400x, H&E staining) Immunohistochemical studies showed negative staining for kappa (C-200x, IHC staining) and positive results for lambda light chain (D-200x, IHC staining)
3. Discussion
Solitary bone plasmacytoma is a localized proliferation of monoclonal malignant plasma cells, considered to be a low-grade progressive form of MM which makes 3% to 10% of all plasmacytomas (1, 5). Gene interactions in the reticuloendothelial system, irradiation overdose, chronic stimulation, and viruses are among the important etiologic factors for plasma cell tumors (13). SBP is more prevalent than EMP (approximate ratio of 2:1) and occurs in male twice as often as in females (1). Patients are more often between 50 to 80 years of age, younger than MM patients (14-16). Skull, long bones, and vertebrae are the most common sites of involvement (6). The presence of SBP in the jaws is extremely rare (4% of cases) and it frequently involves the mandible (2, 4, 11, 14, 17), mostly affecting bone marrow-rich regions such as retromolar trigone, ramus, and angle (2, 4, 18). Criteria for diagnosis of SBP include a single lesion histologically investigated, in the absence of anemia, hypercalcemia, renal insufficiency or multiple lytic bone lesions with negative skeletal survey and tumor-free bone marrow or a very low clonal plasma cell infiltration (19, 20). The diagnosis of SBP in the jaws is difficult and can be misdiagnosed as odontogenic and less frequently malignant tumors in the process. Jaw involvement mostly occurs with pain, dental mobility, paresthesia, bleeding, soft tissue swellings, and sometimes pathological fractures (1, 6, 18, 21, 22). The most common systemic symptoms are fever and fatigue (4, 10, 23). The typical radiographic appearance of SBP is a well-defined solitary ‘‘punched-out’’ lytic area, which may enlarge and appear ‘‘bubbly’’ or separated. Permeative lytic lesions, with poorly-defined outlines, represent rare patterns and are radiographically indistinguishable from skeletal metastases (24). Periosteal reaction is not common and can manifest the early sign of cortical destruction and spreading into surrounding soft tissues (1). CT, MRI and PET scan are used to define the lesion and rule out other sites of involvement (2, 23). Screening of serum or urine by protein electrophoresis should be performed to exclude the possibility of multiple myeloma. Serum Free Light Chain (FLC) assay is a test to identify the quantitation of kappa and lambda chains not bound to intact immunoglobulin molecules in the serum which helps us distinguish SBP from MM (25). In histopathology, diffused and monotonous sheets of neoplastic cells with various types of differentiation (6), from mature plasma cells to undifferentiated cells similar to lymphocyte precursors or intermediate forms are seen in plasmacytoma (6). Malignant plasma cells have eccentric nuclei and stripped nuclear chromatin known as cartwheel with acidophilic inclusions known as Russel bodies (4, 26, 27).
While some authors suggest surgery as the first choice (11, 26, 28) , Radiation therapy (40 – 50 Gy delivered to tumor site) remains as the primary treatment for SBP with local disease control in 80% of the cases (6). Surgical treatment is not the preferred treatment in most instances and remains for patients with well-defined SBPs and minimal cosmetic or functional deficit. It has not been proved that chemotherapy alone halts the progression of SBP to MM but can be used as a combination treatment with radiation for patients with higher risk of treatment failure and tumors greater than 4 - 5 cm (9, 22, 29). As mentioned, our patient was best treated with chemo-radiation without any surgical resection. Bortezomib is a proteasome inhibitor that its clinical efficacy has been established in the treatment of patients with multiple myeloma (30, 31). We believe that this combination therapy may delay the process of SBP converting to multiple myeloma and improve the patient’s survival. It is impossible to know which patient with SBP will eventually progress to MM; therefore, routine laboratory examinations of globulins and monoclonal proteins in serum, creatinine, calcium, Bence-Jones protein in urine, and complete blood count should be included in patients’ follow up routine (11). It is also suggested that urine protein electrophoresis and skeletal survey should be done annually for a period of 5 to 10 years (11).
According to Zachriades et al., jaw plasmacytomas can be the manifestation of MM with an advancement rate of 65% - 100% in 15 years (12). SBP has also a higher chance of being evolved to MM than EMP with a poorer patient prognosis (1, 4, 5, 9, 23, 32). As stated by Tsang et al., a 5-year survival rate for SBP is 60% but it drops to 5.7% if it advances to MM and patients may die from infection, heart and renal failure, bleeding, or thrombosis. It seems that patient’s age (more than 60 years) and size of the lesion (more than 4 - 5 cm) represent higher risk for developing MM and reduce the survival rate (9).
3.1. Conclusion
This was a rare case report of a patient with SBP that occurred in the maxilla and regressed with a combination treatment of chemo-radiation. The patient has been monitored for 18 months since the diagnosis with no signs of recurrence or progression to MM. Early and meticulous diagnosis is crucial to rule out the possibility of a more serious disease of MM which can drastically change the course of treatment in these patients. This progression can worsen the survival rate and therefore there is an important need for constant and close follow up for several years after the treatment.