1. Background
Prostate cancer (PC) is a malignant disease, which is common in men especially in the United States (1). In the initial stages, PC cells need androgens for growth, proliferation, and survival. PC growth can be inhibited by androgen deprivation therapy. But, hormone therapy does not alone cure PC completely because PC cells can progress to androgen-independent phenotype. At this point, PC is resistant to hormone therapy and chemotherapy (2, 3). Studies have shown a significant increase in IL-6 protein level in PC. IL-6 is a pleiotropic cytokine that plays several physiological roles in cells (4). It has been found that IL-6 can involve modulation of growth and differentiation of many cancer cells, such as ovarian cancer, lymphoma, melanoma, and PC (5). Several researchers have shown that IL-6 plays an important role in the progression of PC to androgen-independent phenotype by increasing the expression of androgen receptors in PC cells (6). IL-6 can apply its effects on PC cells by activating several important signaling pathways, such as ERK, erbB1-3, β-catenin, MAPK, Src, JAK-Stat, and PI3k-AKT (7-10). Therefore, it seems that the inhibition IL-6 gene expression and its signaling pathway proteins can reduce the PC cell proliferation.
Gallic acid (3,4,5-trihydroxybenzoic acid) is a polyhydroxyphenolic compound that is extracted from a variety of fruits and vegetables, such as sumac, tea leaves, green tea, apple peels, grapes, and strawberries (11). Several studies have reported that gallic acid has anti-oxidant, anti-inflammatory, and anti-mutagenic properties (11, 12). Some studies have shown that gallic acid can inhibit the growth of cancer cells by inducing apoptosis in cancer cells both in vitro and in vivo (13, 14).
2. Objectives
The aim of this study was to evaluate the effects of gallic acid on IL-6 gene expression, cellular levels of phosphorylated signaling proteins (pSTAT3, pAKT, and pERK1/2), cell viability, invasion, migration, and IL-6 protein secretion in human prostate cancer DU-145 cell line.
3. Methods
3.1. Chemicals
Dimethyl sulfoxid (DMSO), trypan blue, matrigel, and gallic acid were obtained from Sigma (St. Louis, MO). The human prostate cancer DU-145 cell line was purchased from pasteur institute of Iran (Tehran, Iran). RPMI 1640 medium, PEN/STREP, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), and fetus bovine serum (FBS) were prepared from Gibco (Rockville, MD, USA). Antibodies were obtained from Abcam (San Francisco, CA, USA). All other chemicals used were of analytical grade.
3.2. Cell Culture and MTT Assay
DU-145 cells were grown in RPMI 1640 enriched with L-glutamine and 10% FBS, 100 U/mL penicillin and 0.1 mg/mL streptomycin. They were maintained at 37°C in a humidified 5% CO2 incubator. MTT assay was done in order to evaluate the viability of DU-145 cells against gallic acid (15). Briefly, DU-145 cells were seeded in 96-well plates, at a density of 5000 cells/well, and were incubated for 24 hours. After 24 hours, cells were treated with different concentrations of gallic acid (0 - 100 µM) for 48 hours. Then, 100 µL of colorless RPMI 1640 and 10 µL of MTT 12 mM were added to each well and incubated for 4 hours. Formazan crystals were dissolved in 50 µL of DMSO. The viability percentage was calculated by measuring formazan absorbance at 490 nm, using microplate reader (Stat Fax 3200, Awareness Technology, USA) as follows:
Viability = A (sample) / A (control) × 100. The experiments were repeated 3 times.
3.3. Real-Time Quantitative Polymerase Chain Reaction (RT‑qPCR) for IL-6 Expression
IL-6 gene expression was evaluated by RT-qPCR. DU-145 cells were seeded at 2 × 105 cells/well in 96-well plates and were exposed to various concentrations of gallic acid (0, 15, 30, and 35 µM) for 48 hours. Then, cells were harvested and their total mRNA was extracted by Biozol reagent based on the manufacturer’s instructions. For each concentration of gallic acid, total mRNA concentration and quality were assessed by measuring of 260/280 nm absorbance ratio by using NanoDrop spectrophotometer (Thermo-USA). Next, 1 µg of total mRNA was used for the synthesis of cDNA by using a synthesis kit (Takara Bio Inc., Japan). cDNA reverse transcription was performed, using Prime Script™ reagent Kit (Takara Bio Inc., Japan) according to the manufacturer’s protocol. cDNA was amplified during RT-qPCR, using Rotor‑Gene 3000 (Corbett, Australia) in the presence of SYBR® Green PCR Master Mix (Qiagen, Germany) and specific primers for IL-6 (Forward: 5’-AAGCCAGAGCTGTGCAGATGAGTA-3’; Reverse: 5’-TGTCCTGCAGCCACTGGTTC- 3’) and GAPDH (Forward: 5’-ACACCCACTCCTCCACCTTTG-3’; reverse: 5’-CCACCACCCTGTTGCTGTAG-3’) in each reaction. The primers were designed, using Oligo 6.0 software (Molecular Biology Insights, Cascade, Co.) and were confirmed by blast (NCBI). They were purchased from Eurogentec (Seraing, Belgium). The temperature profile for each reaction was adjusted as follows (16): a first denaturation at 95°C for 10 minutes and 35 cycles in a three-step program (including 15 seconds at 95°C, 20 seconds at 60°C, and 25 seconds at 72°C). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize the gene expression data.
3.4. Western Blotting
DU-145 cells were treated with different concentrations of gallic acid (0, 15, 30, and 35 µM) for 48 hours. Then, cells were harvested and subsequently were lysed in ice-cold RIPA buffer (50 mM Tris‑HCl with pH 8, 150 mM NaCl, 1% Triton 100X, 0.5% sodium deoxycholate, 1 mM EDTA, and 0.1% sodium azide) (17). Lysates were incubated at 4°C for 30 minutes and were centrifuged for 10 minutes at 12000 RPM. The protein lysates were measured, using NanoDrop-2000 spectrophotometer and were boiled with an equal volume of loading buffer for 5 minutes. Then, denatured proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and pre-stained blue protein markers (Bio-Rad) were used for molecular-weight determination. Separated proteins were transferred into polyvinylidene difluoride membranes and were placed in blocking solution (2.5% w/v skim milk powder in Tris-buffered saline (TBST) with 0.1% Tween-20) for overnight at 4°C (17). Then, the membranes were washed 3 times in TBS-Tween buffer for 10 minutes. The membranes were incubated with primary anti-bodies against phosphorylated form of STAT3, ERK1/2, and AKT. After 3 hours, the membranes were washed 3 times in TBS‑Tween buffer for 10 minutes, and they were incubated with horseradish peroxidase‑conjugated secondary anti-body for 2 hours. Finally, after 3 times washing, protein bands were revealed by adding BM Blue POD substrate. β-actin assessed as an internal control.
3.5. Measurement of IL-6
DU-145 cells were seeded in 6-well plates and were kept in an incubator. After 24 hours, cells were treated with different concentrations of gallic acid (0, 25, 30, and 35 µM) for 48 hours. Then, the level of IL-6 was measured in supernatants, using an ELISA kit (AViBion Human IL‑6 ELISA kit) according to the manufacturer’s protocols.
3.6. Transwell Migration and Invasion Assays
Invasion assay was done, using transwell with 8 μM pores, 24 well plates, and matrigel. The upper chamber of the transwell was coated with matrigel at a concentration of 1 mg/mL and the lower chamber was filled with 10% FBS-medium as chemoattractan. DU-145 cells were treated with different concentrations of gallic acid (0, 15, and 35 µM) for 48 hours. Then, cells were detached by trypsin and were resuspended in 0.1% FBS-medium. DU-145 cells (5 × 104 per well) were added into the upper chamber of the transwell and they were incubated at 37°C in 5% CO2 for 24 hours. Subsequently, cells on the upper chamber of the transwell were scraped with a cotton swab. Cells on the bottom side of the transwell (migrated cells) were fixed by 5% glutaraldehyde and were stained with 0.5% toluidine blue solution (18). Finally, fixed cells in the low chamber of the transwell were counted, using an inverted microscope. Three independent areas per well were counted and the mean number of migrated cells was calculated.
3.7. Statistical Analysis
The results were expressed as mean ± SD. All statistical analyses were performed, using SPSS version 20.0 software (SPSS, Chicago, IL, USA) and prism 5 software. Group means were compared by Kruskal-Wallis test and P < 0.05 was considered statistically significant. Inhibitory concentration of 50% (IC50) was calculated by the Probit procedure (SPSS software). The analysis of relative gene expression data was estimated with the ΔΔCT method and the data were expressed as fold change. Melting curves were generated to ensure the purity of the amplification product of each reaction.
4. Results
4.1. The Effect of Gallic Acid on DU-145 Cell Proliferation
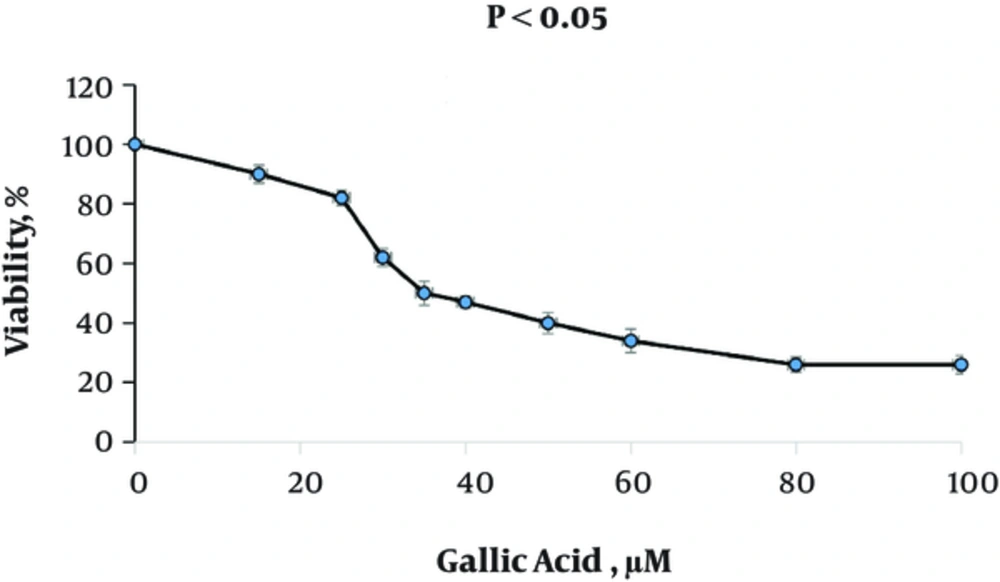
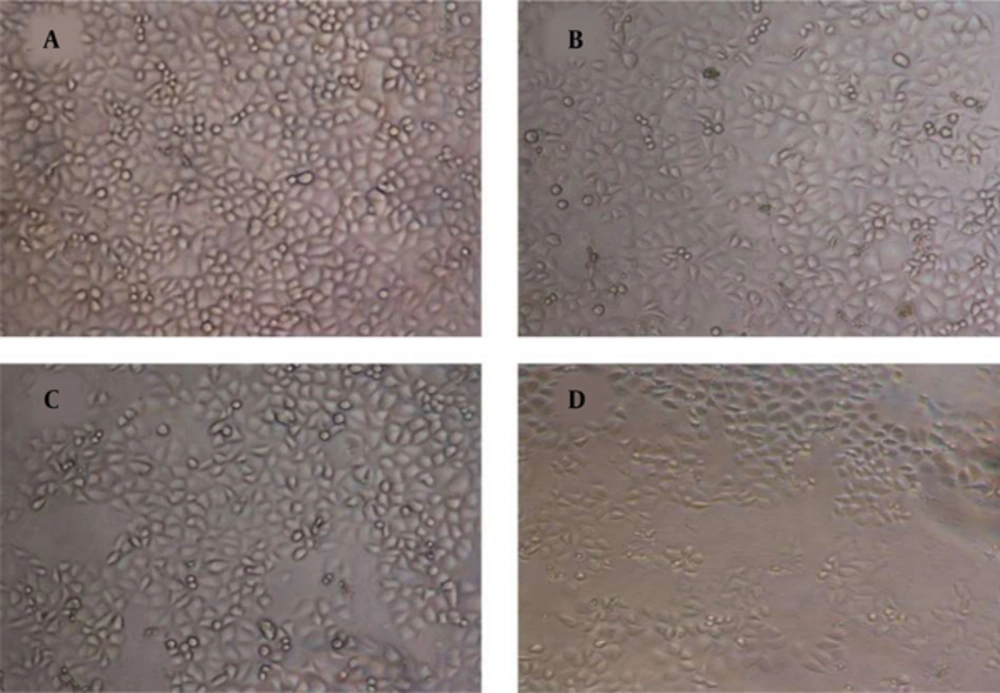
Figure 1 shows the effect of gallic acid in different concentrations on the proliferation of DU-145 cells. Gallic acid gradually decreased the cell viability with the increased concentrations of gallic acid. Treated-DU-145 cells with gallic acid exhibited an IC50 about 35 μM. Also, treatment with gallic acid resulted in morphological changes in DU-145 cells (Figure 2).
Effect of Gallic Acid on Cell Viability and Proliferation of DU-145 Cells; DU-145 cells were seeded in 96 well plates for overnight and, then, were treated with 0 - 100 μM gallic acid in DMSO or DMSO alone for 48 hours. At the end of treatment times, cell viability was measured by MTT assay. Data indicate mean ± SD, n = 3.
4.2. Effect of Gallic Acid on IL-6 Gene Expression in DU-145 Cells
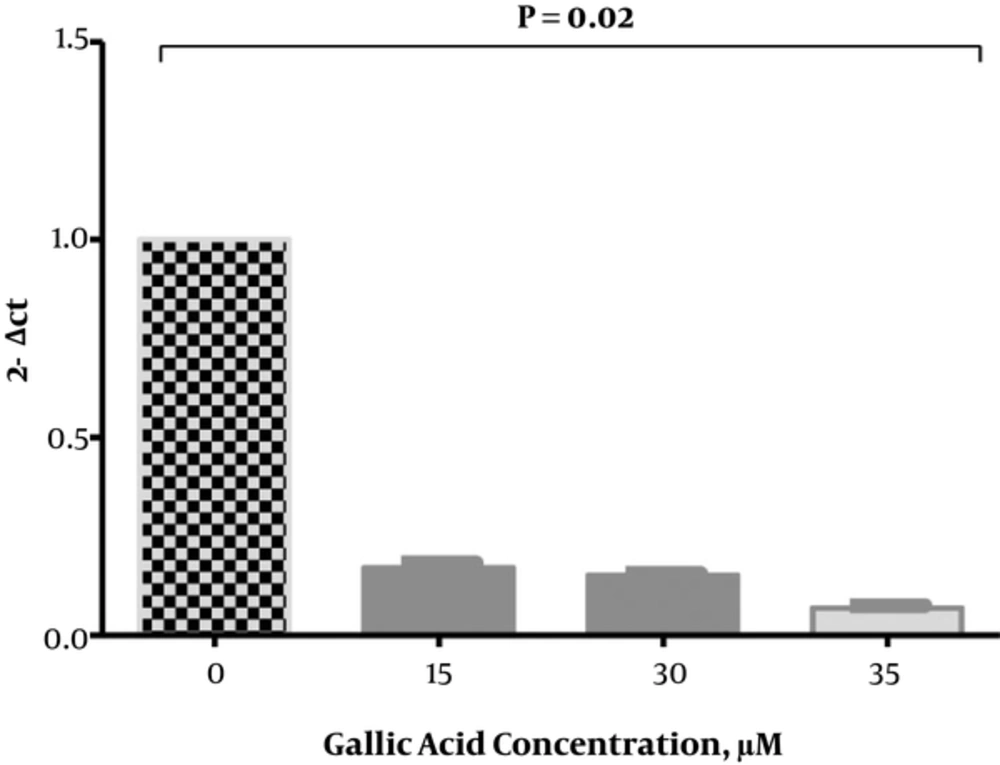
Figure 3 shows the gene expression of IL-6 in treated DU-145 cells with gallic acid compared to untreated cells. Gallic acid significantly reduces the gene expression of IL-6 in DU-145 cells. Reduction of IL-6 gene expression significantly increased in treated DU-145 cells compared to untreated cells by almost 82.96, 84.75, and 93.06 fold at 15, 30, and 35 µM concentrations of gallic acid, respectively.
The Effect of Gallic Acid on IL‑6 Gene Expression; Real‑time quantitative polymerase chain reaction showed the expression of IL‑6 in treated PC3 cells. The expression of IL‑6 was significantly down-regulated in DU-145 cells treated with different concentrations of gallic acid after 48 hours. Columns and bars represent mean ± standard deviation of 3 independent experiments. The expression of IL‑6 normalized with glyceraldehyde‑3‑phosphate dehydrogenase as an internal standard.
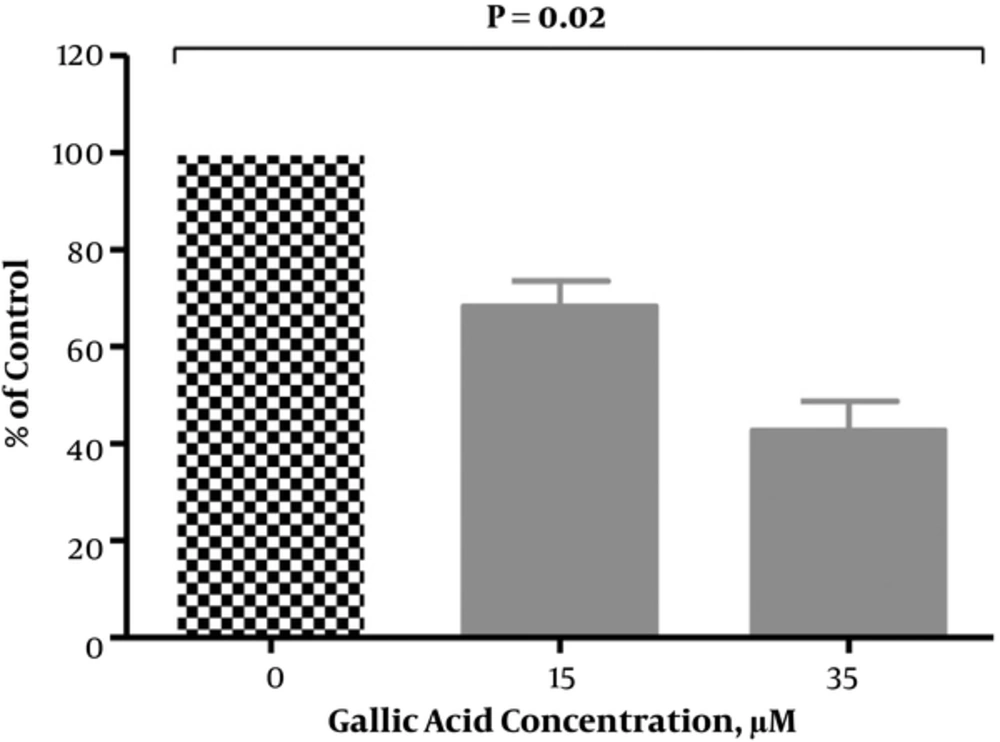
4.3. Effect of Gallic Acid on IL-6 Protein Secretion
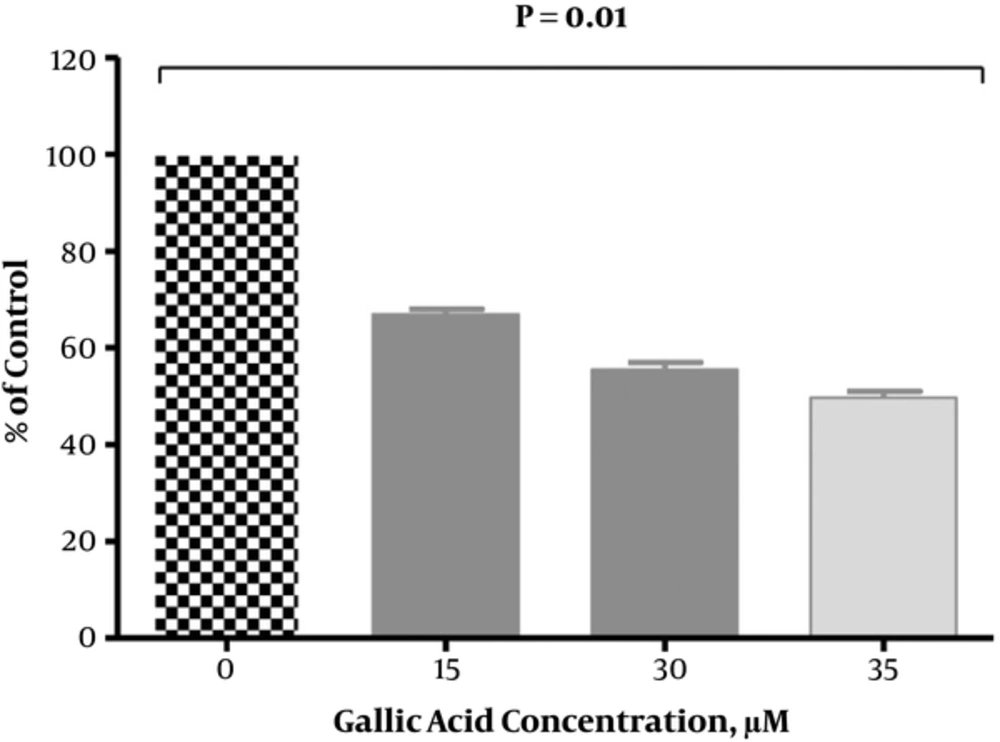
As shown in Figure 4, the level of IL-6 significantly decreased in DU-145 cells that were exposed to gallic acid in a dose-dependent pattern. There was a significant (P < 0.05) reduction in secretion of IL-6 by 33.03%, 44.43%, and 50.29% at 15, 30, and 35 µM of gallic acid, respectively when compared with untreated cells.
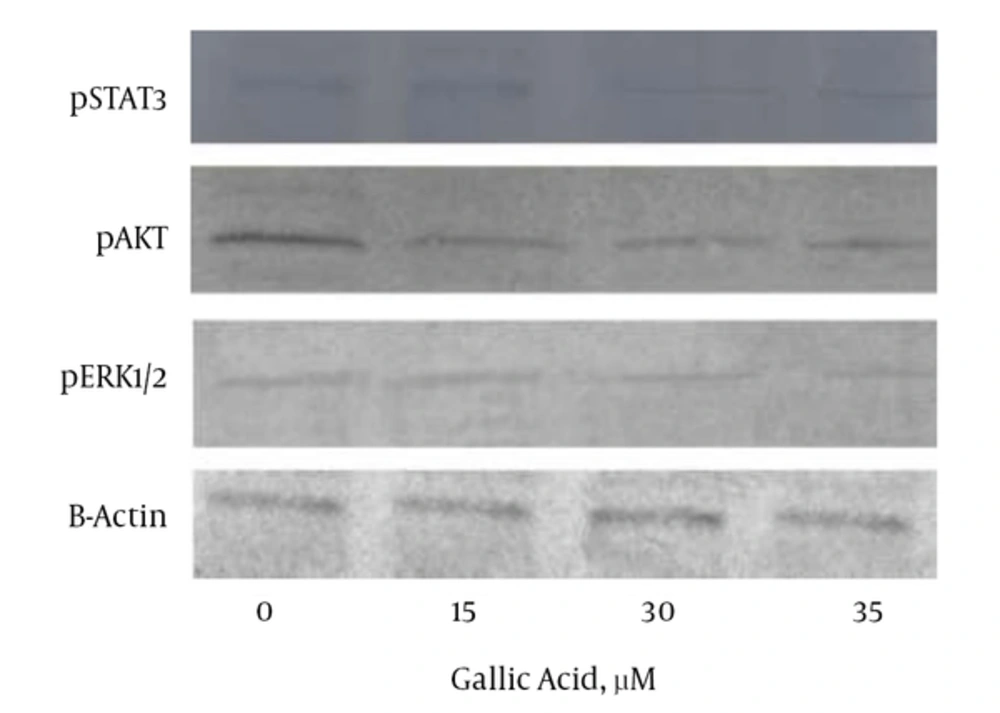
4.4. Effects of Gallic Acid on the Cellular Signaling Pathways in DU-145 Cells
Figure 5 shows that gallic acid led to a decrease in the cellular concentrations of pSTAT3, pAKT, and pERK1/2 signaling proteins in a dose-dependent manner, when compared with the control cells. Treatment with gallic acid at 30 and 35 µM resulted in a more decrease in the level of pERK1/2 than that of pSTAT3 and pAKT. Reduction in the level of pAKT signaling protein caused by gallic acid was lower compared to the decrease of pSTAT3 and pERK1/2 levels.
The Effects of Gallic Acid on the Levels of Signaling Proteins in DU-145 Cells That Were Treated with Increasing Doses of Gallic Acid for 48 Hours; The cell lysates were collected and subjected to Western blot analysis. Gallic acid down-regulated the expression of pSTAT3, pAKT, and pERK1/2. Equal amounts of lysate protein were subjected to gel electrophoresis.
4.5. Effects of Gallic Acid on DU-145 Cells Invasion
According to Figure 6, gallic acid led to a decrease in DU-145 cells invasion in a dose-dependent pattern. Treated DU-145 cells with gallic acid showed a reduction in cell invasion compared to the untreated cells by 31.7 % and 57.3% at 25 and 35 µM, respectively.
The Effect of Gallic Acid on the Invasion of DU-145 Cells; Treated DU-145 cells with different concentrations of gallic acid for 48 hours were placed in invasion chambers for 24 hours at 37°C. Invading cells were counted, using inverted microscope Bars representing the mean ± S.D. of triplicate determinations.
5. Discussion
PC is considered a malignant disease that is common in men (2). Due to the side effects of chemotherapy, multiple studies have focused on the therapeutic potentials of anti-oxidants and plant-derived compounds in the treatment of diseases, especially cancer in recent years (19-23). Previous studies have indicated that gallic acid, as an anti-oxidant agent, can inhibit the growth of PC cells through activating apoptosis (24). In this study, gallic acid especially in IC50 not only decreased DU-145 cells viability, but also changed the morphology of these cells (Figures 1 and 2), which is in agreement with other investigators (25). Also, many studies have shown that gallic acid is an anti-cancer agent with cytotoxic effects against DU-145 cells. It has been demonstrated that gallic acid can block the growth of DU-145 cells at G2/M phases of cell cycle (24). Therefore, in our study, the reduction of viability and proliferation in DU-145 cells, at least in part, may be as a result of anti-proliferative effects of gallic acid that was reported for glioma cell lines in previous study (26). In our study, gallic acid decreased the synthesis of IL-6 in DU-145 cells in dose-dependent manner (Figure 4). Moreover, RT-qPCR confirms the reduction of IL‑6 gene expression after treatment with gallic acid (Figure 3). It has been shown that gallic acid acts as an anti-tumor agent in MDA-MB 231 breast cancer cells by inhibiting the expression of genes that are involved in inflammation, metastasis, anti-apoptosis, and angiogenesis, such as TNF-α, IL-6, IL-8, COX2, and bcl2 (27). Also, numerous studies have shown that gallic acid down-regulated the expression of pro-inflammatory cytokine, such as IL-1β, TNF-α, and IL-6 in both mast cells and rheumatoid arthritis fibroblast-like synoviocytes (FLS) due to its anti-oxidant effects (28). Therefore, it seems that a reduction in the level of protein IL-6 and its gene expression in treated DU-145 cells, at least in part, are related to the ability of gallic acid to inhibit the synthesis of inflammatory factors in these cells due to its antioxidant effects. Previous studies have shown that gallic acid can inhibit the growth of cancer cells by inactivating signaling pathways, such as PI3K/AKT (29), JAK‑STAT (16), and ERK1/2 MAPKs (30), which are in line with our results. Also, there is accumulating evidence that the phosphorylated form of AKT plays a critical role in the growth and survival of PC cells by inhibiting apoptosis (31). Moreover, in another study, it has been found that gallic acid induces apoptosis in fibroblast-like synoviocytes (FLS) from patients with rheumatoid arthritis (RA) through regulating both pAKT and P53 pathways (28). Thereby, reducing the level of pAKT signaling protein, which was observed in our study, at least in part, can be a reason for reducing DU-145 cell proliferation and survival after treatment with gallic acid. In addition, as seen in Figure 5, gallic acid causes a remarkable decrease in the level of pSTAT3 signaling protein in DU-145 cells at IC50. On the other hand, our findings also demonstrated that pERK1/2 signaling pathway was reduced by gallic acid (Figure 5), which are in line with other studies (16, 30). Previous studies have shown that pSTAT3 protein is involved in cell survival through increasing the production of anti‑apoptotic factors, such as Bcl-2 and Bcl-XL (32). It has also been shown that the activation of MAPK/ERK1/2 pathway can contribute to cell survival by transmitting extracellular inflammatory signals into intracellular responses (33). Nevertheless, some studies have also demonstrated that down-regulation of STAT3 expression by inhibiting IL6/JAK1 pathway can inhibit tumor growth in both lung and colon cancers (34, 35). Therefore, in this study, the reduction in viability and proliferation of DU-145 cells after treatment with gallic acid, at least in part, may be the result of a reduction in the active form of pSTAT3, pERK1/2, and pAKT signaling proteins. In this regard, similar results were observed in treated DU-145 cells with andrographolide (16). On the other hand, the results of our study indicated that gallic acid could inhibit the invasion of human prostate cancer cell line in IC50 (Figure 6), which was associated with down-regulation of IL-6 expression. Previous studies have shown that the inactivation or deletions of PTEN (phosphatase and tensin homolog deleted on chromosome 10) can stimulate AKT activation and lead to an increase in metastasis (36). Also, the stimulation of P2Y receptors by P2Y agonists enhances prostate cancer cell invasion through increasing the activity of ERK1/2 and p38 tyrosine kinase (37). However, anti-oxidants can reduce metastasis in DU-145 cells by inhibiting the P38 MAPK and AKT signaling pathways (38). Therefore, it seems that in this study, reduction in invasion of DU-145 cells, at least in part, is related to the decrease in the level of IL-6 and its signaling protein pathways (pSTAT3, pERK1/2, and pAKT) by gallic acid.
In this study, other factors, such as p65, caspase-3, and the levels of regulatory effectors, such as Bcl-2 and Bax were not investigated. These factors can play an important role in cellular mechanism proliferation, survival, apoptosis, and invasion. Therefore, we suggest that future studies focus on the effects of gallic acid on these factors.
5.1. Conclusions
Our results show that gallic acid can lead to a reduction in survival, proliferation, and invasion in DU-145 cell line by reducing protein IL-6 and its gene expression, pSTAT3, pERK1/2, and pAKT signaling protein pathways. Therefore, it seems that gallic acid can be regarded as a potent agent in the treatment of PC.