1. Background
Cancer is an invasive disease resulted from abnormal growth of cells with the potential to invade other parts (organs) of the body (1). Breast cancer is the most common non-skin malignant disease in women (2). Breast cancer after lung cancer is the second cause of death among women. Different genetic and environmental factors affect the risk of developing breast cancer (3). Triple-negative breast cancer (TNBC) refers to cancerous cells lacking estrogen, progesterone, and Her2/neu receptors. The possibility of reoccurrence after treatment is high in TNBC (4). The MDA-MB 231 cell line of breast cancer is known as a TNBC cancer cell line with stem-like characteristics (5).
Alteration of physiological pathways is common in cancers. Some paths, such as those related to apoptosis and proliferation are disturbed as well in TNBC (6). Such changes increase the potential of growth, local and distant invasions. Hence, studying these pathways and their changes are crucial in cancers.
The hedgehog signaling pathway has a central role in the processes of growth, differentiation, and development of biological systems (7). This signaling pathway is involved in cellular destination, epithelial to mesenchymal cells (EMT) process, and cell proliferation by a change in the adherence and motility of cells. This pathway participates in the safety of stem cells, tissue repair, and homeostasis in adults. Therefore, disruption in this pathway could cause physiological disorders and tumorigenesis in tissues (8, 9). The hedgehog signaling pathway includes a few main members which are kept conserved during evolution in various animals. Patched1 protein receptor (PTCH1) is one of the components which span the cell membrane 12 times and during the absence of hedgehog ligand inhibits this signaling pathway. The inhibitory effect of PTCH1 on the smoothened protein receptor (SMO) is lost when the hedgehog ligand connects to PTCH1. The SMO has seven zones through the cell membrane and is a member of the big group of G-protein connecting receptors (GPCR). The result of interaction activates SMO and then GLI transcription factors in vertebrates. These transcription factors essentially control the gene expressions of certain genes (10).
Quinacrine is a derivative of 9-aminoacridine. It has three heterocyclic rings. At first, it was used as an anti-malarial drug. More studies showed it could be used as an anti-cancer drug by inhibiting signaling pathways necessary for cancer cell activity (11). This drug activates P53 and inhibits PI3K/AKT/mTOR and NF-κB. It is shown that this drug decreases the expression of Bcl2 and Bcl2L1 anti-apoptosis proteins and increases Bax protein (12).
The impacts of quinacrine on the hedgehog signaling pathway and the proliferation of triple-negative breast cancer cells have not been well studied yet and elucidation of such effects may shed new light on the mechanism of quinacrine effects on cancer cells.
2. Objectives
The goal of this study was to evaluate the effect of quinacrine on PTCH1 and SMO genes expression as the major role player of the hedgehog signaling pathway in MDA-MB 231 breast cancer cell line.
3. Methods
3.1. Cell Culture
In this experimental study, MDA-MB 231 breast cancer cell line was obtained from the National Cell Bank of Iran (NCBI). Cells were cultured at 37°C, 96% humidity and 5% CO2 in Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (final concentration of 100 IU/mL and 100 µg/mL, respectively).
3.2. MTT Assay
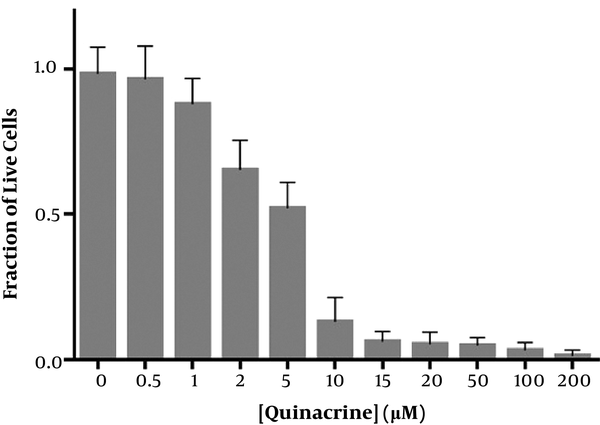
3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium (MTT) assay was used to measure the toxicity of quinacrine (Sigma, St. Louis, USA) on the cells. Cells were treated with concentrations of 0.5, 1, 2, 5, 10, 15, 20, 50, 100, and 200 µM quinacrine for 72 hours and IC50 and IC5 were calculated by Prism GraphPad 6 software. The concentration of 0.5 µM quinacrine for 72 hours was selected for the rest of the study.
3.3. RNA Extraction and cDNA Synthesis
Total RNA was extracted from control and treated cells (72 hours and 0.5 µM quinacrine) using the CinnaPureTM kit (CinnaGen, Tehran, Iran). The adsorption ratio of 260.280 and 1.3% agarose gel electrophoresis was applied to assess the qualifying and quantify of RNA and possible DNA contamination. DNase I kit (Life Technologies GmbH/ThermoFisher Scientific, Darmstadt, Germany) was used to eliminate the DNA impurity. The cDNA synthesis was done by RevertAid First Strand cDNA Synthesis Kit (Life Technologies GmbH/ThermoFisher Scientific, Darmstadt, Germany) based on the protocol of manufacture.
3.4. Quantitative Real-Time PCR (qRT-PCR)
PTCH1, SMO, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene primers were designed using primer blast from NCBI (https://www.ncbi.nlm.nih.gov/nuccore) (Table 1).
| Gene | Primer (5’ → 3’) | Product Size |
|---|---|---|
| GAPDH | 116 | |
| F | GGTCTCCTCTGACTTCAACA | |
| R | AGCCAAATTCGTTGTCATAC | |
| PTCH1 | 177 | |
| F | TCAGAGACTGGCTTCAGGGA | |
| R | GACGCTGTTTAGTCAACTGGC | |
| SMO | 132 | |
| F | CCCTGGTCTCCAACCCATTC | |
| R | GGTTGGTGCGGGAGTGAATA |
Primers Sequences of PTCH1, SMO and GAPDH Genes
The expression of selected genes was measured using SYBR Green quantitative real-time PCR by using the Rotor-Gene 6000 real-time PCR machine (Corbett). Real-time PCR amplification was started with an initial predenaturation cycle of 95°C for 4 minutes, followed by 40 three‐step amplification cycles (95°C, 10 seconds; 60°C, 20 seconds; 72°C, 15 seconds) and was completed with the subsequent melting curve analysis. LinReg software was applied to calculate the amplification Ct and efficiency. The Ct values obtained for each case were evaluated comparing with Ct value for the GAPDH gene. This gene was the reference gene in this study. The relative expression of target genes was calculated by the Pfaffl method (13). Electrophoresis of the PCR products was performed by agarose gel.
3.5. Statistical Analysis
Experiments were performed in triplicate. For all quantitative data, mean value ± one standard deviation (SD) was presented. A Student’s t-test was used and calculated P value less than or equal to 0.05 was considered as a statistical significance.
4. Results
Quinacrine revealed an IC50 of 3.5 µM for MDA-MB 231 cells for 72 hours of treatment (Figure 1). Therefore, nontoxic dose of 0.5 µM quinacrine (IC5) was applied to investigate the effect of quinacrine on genes expression.
RNA was extracted after treating cells for 72 hours with 0.5 µM quinacrine. The samples had a 260/280 absorption ratio in the range of 1.8 - 2. In agarose gel electrophoreses, 28S rRNA and 18S rRNA bands were observed in control and quinacrine treated cells.
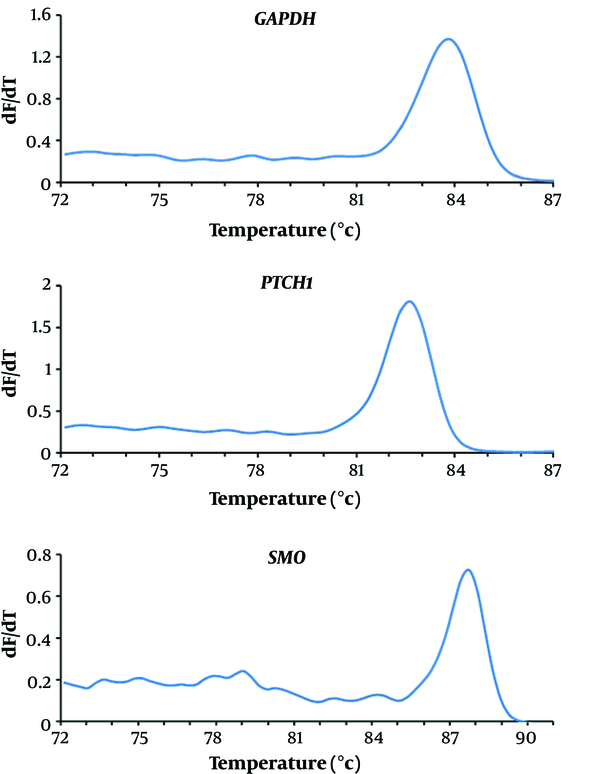
Melting curve evaluation of the amplification of GAPDH, PTCH1, and SMO genes showed the specificity of the selected primers for genes expression evaluation (Figure 2). Agarose gel electrophoresis of PCR products is shown in Figure 3.
Quinacrine significantly decreased (fold: -2.1 ± 0.1 and P = 0.007) SMO gene expression in treated MDA-MB 231. However, PTCH1 gene expression did not show a statistically significant difference (P = 0.059) (Figure 4).
Alterations in the PTCH1 and SMO genes expression in MDA-MB 231 cells treated with 0.5 µM quinacrine for 3 days. Graphs represent mean fold changes ± SD for at least three replicates, and the significance of differences was defined by t-test (P < 0.05). Significant downregulation of SMO (fold: -2.1 ± 0.1 and P = 0.007) expression is evident in treated cells but not change the expression of PTCH1 (P = 0.059) is apparent in treated cells (* P < 0.05).
5. Discussion
In this study, quinacrine decreased SMO gene expression in breast cancer MDA-MB 231 cell line while PTCH1 gene expression was not changed. Breast cancer, which is recognized as the most common non-cancerous malignancy in women, can be expressed in different forms based on different molecular criteria expressed in it (2). These forms can vary in the terms of invasion rate, the type of cells involved, and the clinical conditions of patients (14). One of the most important types of breast cancer is breast cancer with a triple-negative profile that does not contain estrogen, progesterone, and HER2 receptors (15). The prevalence of this type of breast cancer in some parts of Iran is about 34%. Patients with this type of breast cancer are typically less than 40 years old (16). This kind of breast cancer is associated with a worse prognosis and does not respond to typical treatments and has the morphology of epithelial cells with substantial invasion (17). In this study, MDA-MB 231 cell line was used to evaluate this type of breast cancer. These cells have the characteristics of triple-negative breast cancer cells, which are highly invasive (18).
In recent years, signaling pathways have been considered by the researchers as a therapeutic goal in treating cancer. One of the most controversial of these pathways is the hedgehog signaling pathway (19). This pathway can induce the essential characteristics of cancer cells by creating various molecular mechanisms (20). Since the misplaced activity and out-of-control of the hedgehog signaling pathway is accompanied by tumorigenesis and tumor growth by various mechanisms, this cascade signaling pathway provides a new target for cancer treatment (8). Mutations in the main members of this signaling pathway are one of the mechanisms for the development of cancer through this pathway (7).
The hedgehog signaling pathway is composed of the PTCH-SMO-GLI axis. The SMO protein has a critical role in this pathway. This protein can affect the activity of the GLI transcription factors (21, 22). Considering these conditions can lead us to select the most appropriate classification of inhibitors of this pathway. Currently, the most advanced factor targeting the hedgehog pathway is vismodegib, approved by the FDA, and used to treat metastatic basal cell carcinoma (BCC). Vismodegib acts as a competitive antagonist for SMO. Other inhibitors of this pathway that are linked to the SMO include saridegib and cyclopamine. Typically, the use of cyclopamine and vismodegib, which act as an inhibitor of the hedgehog pathway, is associated with drug resistance. The reasons for this drug resistance include the presence of a mutation in the binding site of the drug to the receptor, an increase in the GLI2 expression, and interactions created between the other signaling pathways with the GLI1 transcription factor (23).
The effect of quinacrine, a derivative of 9-aminoacridine, has been studied in some cancers such as breast cancer in recent years. The anti-cancer effect of this drug is performed in a complicated manner (24). In the present study, quinacrine was applied due to its ability on various signaling pathways that affect the death and survival of cancer cells (25). It has been shown that quinacrine can hinder some of the target genes expression of this signaling pathway by binding to the GLI-DNA complex in cervical cancer stem cells (26). In this study, a concentration of 0.5 µM of quinacrine was used. It has been shown that quinacrine does not have a significant toxic effect on normal breast cells in this concentration (27). Cells were treated with quinacrine for 72 hours to evaluating the effect of quinacrine on gene expression.
In the present study, it was found that quinacrine significantly reduces the SMO gene expression in MDA-MB 231 breast cancer cell line. This gene is an oncogene. The reduction of the expression of this gene can be added to the anti-cancer effects of quinacrine on triple-negative breast cancer. The SMO protein content was not evaluated in this study. It is suggested that the effect of quinacrine should be investigated on the amount of SMO protein in MDA-MB 231 breast cancer cell line.
5.1. Conclusions
In conclusion, the results of the present study indicate that quinacrine in the concentration of 0.5 µM has anti-cancer effects in human breast cancer triple-negative cells through the effect on the SMO member of the hedgehog signaling pathway. The more detailed mechanism of quinacrine on the hedgehog signaling pathway in the MDA-MB 231 breast cancer cell line remains to be elucidated.