1. Introduction
Squamous cell carcinoma (SCC) accounts for the majority of uterine cervical cancer cases (1). According to WHO’s classification, histologic variants of cervical SCC include keratinizing, non-keratinizing, basaloid, verrucous, warty, papillary, lymphoepithelioma-like and squamotransitional (2). Among different histologic subtypes, basaloid squamous cell carcinoma (BSCC) tends to behave in an aggressive and highly invasive fashion. Surprisingly, the uterine cervix is among the rarest sites for occurrence of such infrequently confronted pathology; however, a few well-documented examples are on the record (3, 4). We aimed to report a basaloid squamous cell carcinoma of cervix in a woman with previous subtotal hysterectomy.
2. Case Presentation
A 62-year-old woman, gravid 9 and para 8, postmenopausal from 8 years prior to the study was referred to our hospital, having complained of post-menopausal bleeding for the past 6 months. She mentioned a history of subtotal hysterectomy due to postpartum hemorrhage that occurred following a normal vaginal delivery 26 years earlier. The pathologic report was unremarkable once more. She had not participated in cervical cancer screening program, Pap smear in particular. Her social history was positive for passive household smoking but she denied any of the alcohol consumption or sexual promiscuity.
In genital physical examination, cervix was 4 centimeters in diameter and there was an ulcerated punched out tumoral lesion. Left parametrium was short and thick whereas right parametrium and rectovaginal septum were both normal-appearing. In the general examination, there was no lymphadenopathy in inguinal or other regions. Colposcopy assisted biopsy revealed a diagnosis of squamous cell carcinoma. As far as clinical staging was concerned, the tumor was FIGO (Fédération Internationale de Gynecologie et d’Obstétrique) stage II. Metastasis workup was negative and laboratory tests were within normal ranges. Pelvic magnetic resonance imaging (MRI) showed an enhancing heterogeneous mass measuring 24 × 18 mm located in the cervix. Therefore, in a preplanned procedure, the patient underwent trachelectomy, and bilateral lymph nodes dissection. The surgical specimens were put in formalin and were sent to central pathology lab.
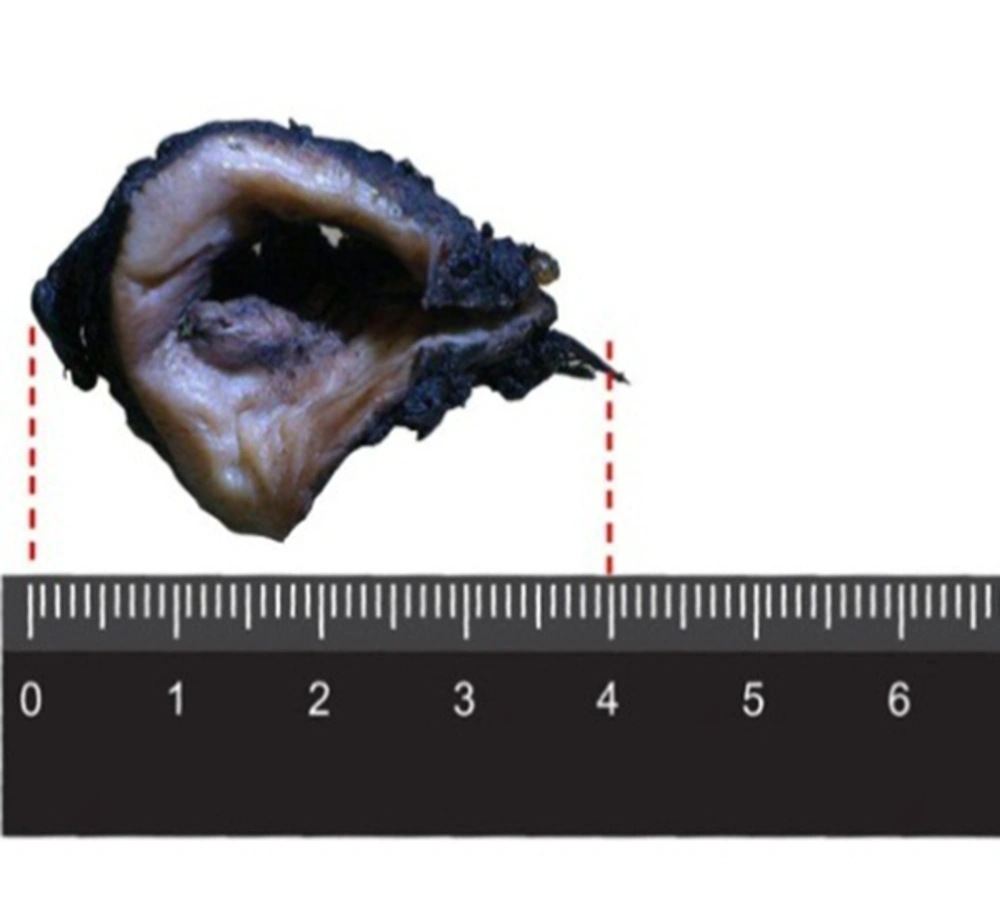
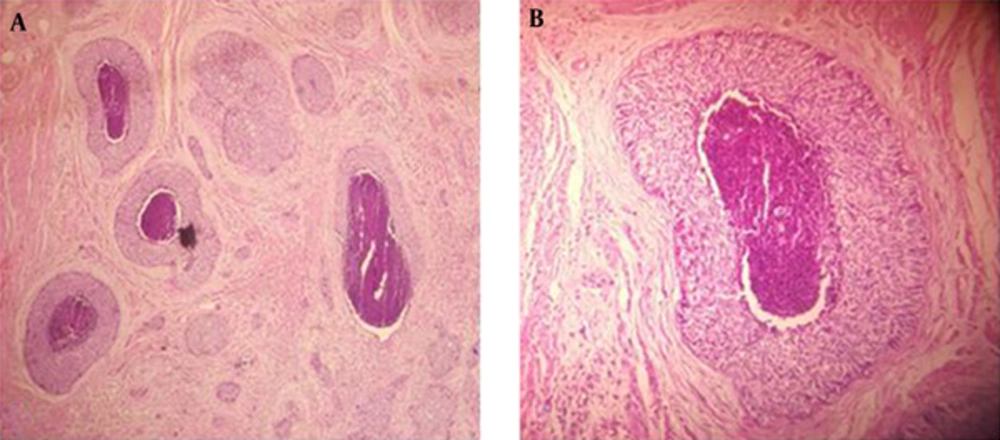
The specimen on gross examination compassed a pale- tan ulcerative and infiltrative mass measuring 3 × 2 cm involving the entire thickness of the cervix (Figure 1). In addition, light microscopic examination with H and E staining suggested that the cervix uteri was infiltrated by neoplastic tissue that formed nests of small, round to oval-shaped cells, scant cytoplasm, hyperchromatic nuclei with prominent peripheral palisading pattern containing foci of comedo-type necrosis (Figure 2). Both lymphovascular space and perineural invasion were also noted. The distal surgical margin located at vaginal cuff was microscopically clear, while left parametrium was involved by tumor. No evidence of tumoral involvement was detected in 20 dissected lymph nodes.
The immunohistochemistry examination revealed that the tumor was positive for cytokeratin AE1/AE3 (CK AE1/AE3) and negative for p63, C-KIT, chromogranin and synaptophysin markers. In addition, strong and diffuse cytoplasmic, and nuclear p16 expression were seen in neoplastic cells. Thus, the final pathologic diagnosis was basaloid squamous cell carcinoma of uterine cervix.
The patient was consulted for adjuvant radiotherapy because of parametrial involvement. After the completion of radiotherapy, the patient was put under close follow-up, consisted of history and physical gynecological examination, every 3 months within the first two years and other imaging or laboratory tests as indicated. The follow up visits were uneventful within the 24 months after surgery.
3. Discussion
Cervical cancer includes a heterogeneous number of varieties with completely different biological and histological characteristics (5). Basaloid squamous cell carcinoma (BSCC) is mainly an uncommon but aggressive type of squamous cell carcinoma. This malignant neoplasm generally affects women in their 60 seconds and 70 seconds as our case, although there are occasional reports of occurrence in younger individuals (6, 7). Most BSCCs are located in the upper aerodigestive tract, but they can develop anywhere throughout the body, including lung, vulva, vagina and cervix (8). Human papillomavirus (HPV), serotypes 16 and 18 in particular, is commonly associated with conventional squamous cell carcinoma as well as other types such as basaloid and warty variants (9). Accordingly, in the present case, strong and diffuse expression of p16 was an evidence for the role of HPV. Although smoking and alcohol consumption are supposed to have a strong association with this tumor (7), our patient denied such history.
Our patient had a history of subtotal hysterectomy that compared with total hysterectomy is quicker, simpler with less intraoperative blood loss, and fewer overall perioperative complications (10). The incidence of cervical stump carcinoma with such history is 1% - 3% (11). Squamous cell carcinoma is the most common histology of cervical stump cancer followed by adenocarcinomas (12). The mean interval between subtotal hysterectomy and diagnosis of the cervical stump was reportedly 17.6 years (11). The prognosis of stump cancer is worse than intact uterus (13). Delay in diagnosis is not uncommon due to lack of proper screening. In our patient, screening test after subtotal hysterectomy was not performed and BSCC occurred 26 years after subtotal hysterectomy.
The differential diagnosis between BSCC and other entities may be difficult including basal cell carcinoma, spindle cell squamous carcinoma, adenoid cystic carcinoma, adenosquamous carcinoma, cystic, small cell carcinoma, large cell neuroendocrine carcinoma of the cervix and basosquamous cell carcinoma (9, 14-16). In this case, the microscopic features of BSCC were nests of small, round to oval-shaped cells, scant cytoplasm, hyperchromatic nuclei with prominent peripheral palisading and foci of comedo necrosis. The apparent morphological characteristics generally make this differentiation easy, but when in doubt, several sections might be provided to ensure accurate categorization. In certain cases, immunohistochemistry can come in useful to unveil the correct diagnosis. The accurate diagnosis is mandatory since BSCC varies in biological behavior, prognosis and therapeutic approach from entities mentioned above (9). In the same manner, application of immunohistochemical (IHC) markers helped us diagnose this tumor. Previous studies showed that BSCCs are positive for CK-AE1/AE 3, cytokeratin-7 (CK7), tyrosine-protein kinase (C-KIT), Carcinogenic Embryogenic Antigen (CEA), Cancer Antigen 19-9 (CA19-9), Epithelial Membrane Antigen (EMA), cytokeratin-34βE12 (CK 34BE12) whereas negative for p63 protein, chromogranin, synaptophysin, platelet derived growth factor receptor (PDGFRA), estrogen receptor (ER), cluster of differentiation-56 ( CD56), and thyroid transcription factor-1(TTF-1) (7, 17). In agreement with earlier studies, the tumor cells in our case stained negative for P53, chromogranin, synaptophysin while positive for CK AE1/AE3 and EMA.
The aggressive natural behavior of BSCC has been generally related to early recurrence, lymph node involvement and also distant metastasis to the liver and lung (18). Lymphatic involvement was not observed in our patient. However, there are no prior studies comparing stage-by-stage outcome and survival in basaloid squamous cell carcinoma versus usual squamous cell carcinoma of the cervix.
Based on above, basaloid squamous cell carcinoma although it is rare, it should not be kept far from a pathologist mind already knowing the clinical behavior of this entity differs from other SCC subtypes. Light microscopy along with IHC is the backbone of diagnosis.