1. Context
The prevalence of gliomas is approximately 50% among all brain tumors and malignant gliomas account for 80% of all brain malignancies (1, 2). These types of brain tumors are categorized into grades I to IV according to World Health Organization (WHO) classification (3). Gliomas grade II or III are very aggressive and the probability of tumor progression is much higher and has a poor prognosis (4, 5). The highest malignancy rate belongs to grade IV gliomas, known as glioblastoma multiforme (GBM); it has a high proliferation with high adhesion to the surrounding tissue and very hard to remove by en bloc resection, which, in turn, deteriorates the condition (1, 3). Predominantly common risk factors for gliomas are genetic predisposition and previous exposures to radiation, while many of them may occur spontaneously (2). Some evidence also suggests that obesity, vitamin D deficiency, and atopy are causative factors for gliomas risk (2, 6, 7). The median survival rate for patients with GBM is approximately 12 to 15 months and despite the full implementation of treatment protocols such as surgery, radiotherapy, and chemotherapy, it remains incurable (1-3). Therefore, adjuvant therapies seem to be necessary. Recently, vitamin D is known as a neurosteroid hormone (8, 9). Hence, it can play varied roles in the brain. Following the exposure to sunlight, 7-dehydrocholesterol in the subcutaneous tissue is turned to cholecalciferol. This is, then, once hydroxylated in the liver becoming 25-hydroxycholecalciferol. Finally, hydroxylation again occurs in the kidneys and 1,25 dihydroxycholecalciferol or calcitriol, which is an active form of vitamin D3, is produced. The other way is to get vitamin via diet or dietary supplements (10, 11). Beyond the calcium balance role of vitamin D and the beneficial effects on bone, due to the existence of vitamin D receptor (VDR) throughout body especially in the brain, vitamin D regulates the various types of function such as cell proliferation and differentiation, apoptosis, angiogenesis, cell invasion, DNA repair, metabolism, and inflammation (12-14). Although antitumor efficacy of vitamin D3 is observed in supraphysiological doses, which in turn may cause hypercalcemia, recent in vivo studies have shown that its analogs have beneficial effects on glioma cells without calcemic effect (15-18). Here, we review studies about the effect of vitamin D3 and its analogs on gliomas with special emphasis on GBM. Because the number of studies on human subjects (19) did not reach the required minimum for a systematic review, we systematically reviewed studies with in vitro and in vivo design.
2. Evidence Acquisition
The electronic databases, including PubMed, ScienceDirect, Web of Science, Google Scholar, and the Cochrane Central Register of Clinical Trials (last updated April 12th, 2019) were searched, using medical subject headings (MeSH) keywords “vitamin D AND brain malignancy”. The full strategy of the search is shown in supplementary file Appendix 1. Abstracts from recent international conferences and references of the found articles were also searched for additional related articles. Two investigators independently reviewed the found articles at the title and abstract level. Then, the articles that appeared to be relevant were studied in full-text form. Considering PICOS criteria, articles were included in our study based on the following characteristics: (1) population; in vitro and in vivo samples from humans, rats, and mice with malignant gliomas, (2) intervention; the administration of vitamin D3 and its analogs, (3) comparison intervention; placebo-control, (4) outcomes; self-renewal ability, cell viability and/or cell growth, migration rate and invasive capacity, autophagy, and cell death, and (5) study design; in vitro and in vivo studies. There was no limitation on the date and language of the published articles. Molecular and genetic-based studies that were different from the present study in terms of our main predefined objectives, studies focusing on other living creatures, and studies having different design focusing on other cancers were excluded from the study. Studies that covered all inclusion criteria were selected. In terms of the presence of bias, all eligible studies were evaluated, using the GRADE (20, 21) and CIRCLEs RoB tools (22) for in vitro and in vivo studies, respectively. Given that there are no well-known tools and methods for assessing the quality of in vitro studies, we modified the GRADE tool for in vitro studies like other studies (23, 24). Accordingly, we categorized the articles as “high”, “moderate”, “low”, or “very low” quality based on their methods. For in vivo studies, we rated the articles by “yes”, “no”, or “unclear”. Our work is in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (25).
3. Results
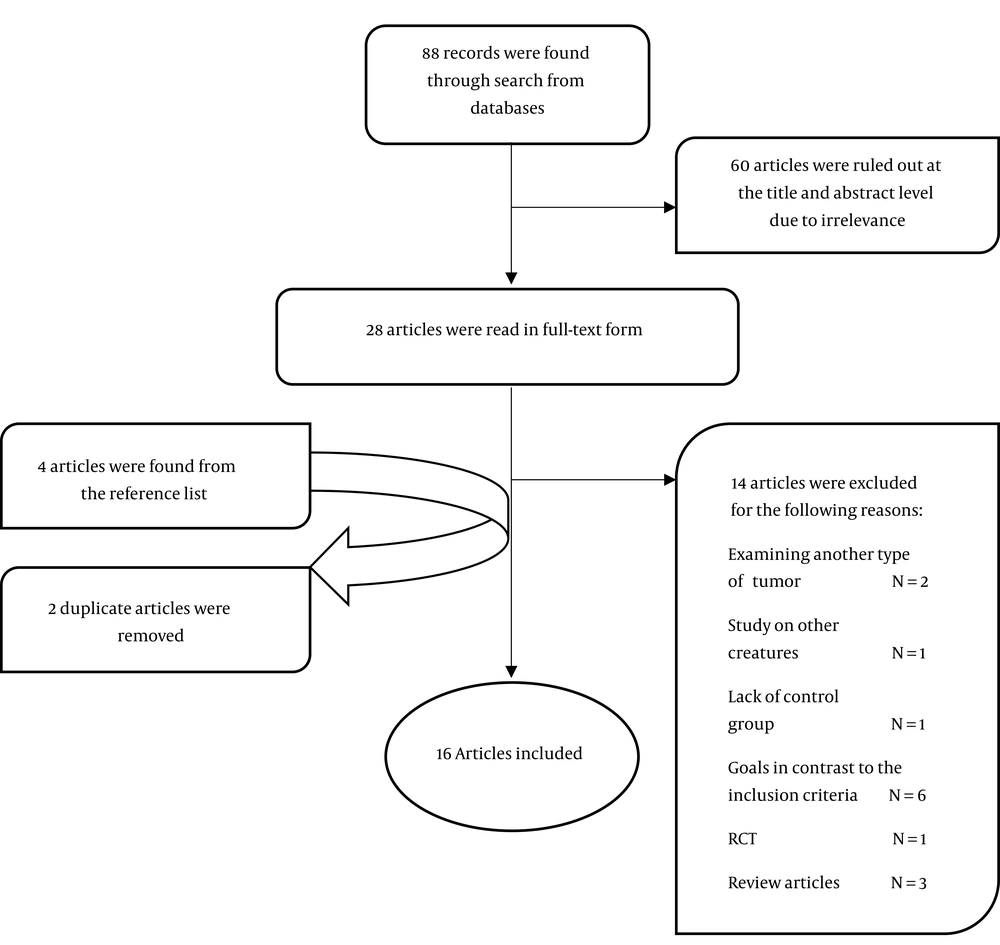
According to the search strategy described previously, 88 papers were found (Figure 1). Twenty-eight articles with the closest characteristics to the inclusion criteria were selected through reviewing the total articles found at the title and abstract level. Then, these articles were examined more precisely. Two articles were excluded since they focused on tumors rather than gliomas (26, 27). In addition, the reasons for removing the rest of studies excluded from present study are as follows: 1 article studied the insects (28), 1 article did not have control group (29), the objectives and outcomes of 6 studies were different from the predetermined aims of the present study (30-35), 1 article had a randomized clinical trial design (19), and 3 studies were review articles (3, 36, 37). Following the further search, 4 articles were found from the reference list, all of which were eligible in terms of inclusion criteria (1, 38-40). Two out of the 4 articles were already found in our search (38, 39), and 2 other articles were new, which are now included (1, 40). Finally, 11 in vitro studies (Table 1) and 5 in vivo studies (Table 2) were included in this systematic review. In terms of bias quality, given that GRADE scoring was used for quality assessment (Table 3), these studies were mainly of moderate to high quality. To evaluate the quality of animal studies, we used SYRCLE’s RoB (Table 4). None of these studies provided a complete description of allocation, randomization, and blinding.
| No. | Compound | Cells/Animal | Culture | Evaluation | Treatment | Main Results | Effectively | Control | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Tacalcitol, calcipotriol, calcitriol | Human glioblastoma cell line T98G | 6 days; For cell migration and caspase activity; 24 hours LDH release; 72 - 120 hours. 48 hours for Assay of 3 H-thymidine incorporation | MTT assay, manual cell counting using a Hemocytometer, Wound healing assay, Measurement of LDH release using Cytotoxicity Detection, Caspase-Glo® 3/7 assay, RT-PCR, Assay of 3 H-thymidine incorporation | 1 nmoL - 10 μmoL | Calcitriol and both its analogs decreased cell viability and/or growth, dose-dependently, mainly inhibited proliferation and reduced the migration rate | Effective | Ethanol | Emanuelsson et al. (17) |
| 2 | Calcitriol | Human GBM cell lines U251, U87MG and T98G, and human breast adenocarcinoma cell line T47D | For cell survival assay 96 hours, 17 hours to cell migration and 48 hours for Immunofluorescence | Tissue microarray construction, Immunohistochemistry, Transfections were carried out, using Lipofectamine 2000, Western blotting (WB), the cells were counted manually, using a hemocytometer, wound healing assay, RNA extraction, and real-time qPCR, Immunofluorescence (IF) | 1 µM | Calcitriol increased the expression of VDR in tumor tissue. VDR expression was correlated with an improved outcome in patients with GBM. Treatment with calcitriol decreased the migration rate of T98G cells and retarded their survival. | Effective | Isopropanol | Salomon et al. (41) |
| 3 | Calcitriol, trichostatin A (TSA), 5-azacytidine (5-aza) | GBM cell lines, Tx3868, Tx3095, U87MG, U118 and U373MG | 24, 48, and 72 hours | Colorimetric immunoassay, Western blotting, PCR, and flow cytometry | 10-6 - 10-10 M calcitriol, 5 - 20 ng/mL TSA, 0.1 - 5 µM 5-AZA | Treatment did not result in significant antiproliferative effects. Treatment had not modulatory effects on the expression of key components of the Notch-signaling pathway. Combination therapy with Calcitriol plus TSA or 5-AZA reduced TSA or 5-AZA antiproliferative properties. | No effective | Ethanol | Reichrath et al. (42) |
| 4 | Calcitriol, Calcidiol | TX3868 and TX3095 were established from glioblastoma and xenografted on mice, COS-1 cells and rat GBM cell line C6 | 6 days | WST-1 Reagent, nested touchdown reverse transcription-PCR (RT-PCR), real-time RT-PCR, high-performance TLC | 10-12 to 10-6 mol/L Calcitriol and Calcidiol in a dose of 2.5 × 10-8 mol/L | 16 splice variants of CYP27B1 in GBM with different expressions between tumor and normal tissues were found, Calcitriol had a proliferative effect on some human GBM cell lines, Calcidiol had the antiproliferative effect in 6 cell lines and proliferative effect on the remaining three cell lines. | Calcitriol was without the expected effects. Calcidiol was effective | Ethanol | Diesel et al. (43) |
| 5 | Cholecalciferol, C2-ceramide | Human glioblastoma cell line Hu197 | 6 and 12 hours | Light microscopy, DNA extraction & pulsed field gel electrophoresis MTT, assay, and lipids extraction & purification | 50 µg of ceramide or 50 µg of cholecalciferol | Cholecalciferol treatment was increased by intracellular ceramide and induced cell death. Ceramide also resulted in induced cell death in the same cells | Effective | Same cells without any intervention | Magrassi et al. (44) |
| 6 | Calcitriol | The clones C6.9D3Sen and C6.4 D3Res were isolated from the C6 rat glioma cell line | 24 hours | Plasmid construction &transfections, MTT assay, DNA fragmentation, southern blot, northern blot analysis, biorad protein assay, radiolabelled ligand assay | 10-11 to 10-7 M | Treatment reduced cell number compared with control cells | Effective | Ethanol | Davoust et al. (38) |
| 7 | Calcitriol | Rat glioma C6 cell line | 24 hours | cDNA library and high-density filters, hybridization and differential screening analysis, DNA sequencing and computer analysis, RNA isolation for Northern blot analysis and cDNA probe preparation, evaluation of the length of inserts | 5 × 10-8 M | Calcitriol has reduced the expression of PMP22/gas3, SPARC/ON, MAP1C/dynein heavy chain, S100β, and aldolase C genes, and increased cysteine-rich protein, MGP, β-Tubulin, mortalin | Effective | Ethanol | Baudet et al. (45) |
| 8 | Calcitriol | Rat glioma C6.9 and C6.2 cell lines. | 24 hours | MTT Assay, DNA Isolation & Analysis, RNA Isolation & Northern Blot, DNA Flow Cytometry, Electron Microscopy | l0-7 M | Cell death induced by calcitriol was depended on the synthesis and expression of genes such as c-myc, p53, and gadd45 | Effective | Ethanol | Baudet et al. (40) |
| 9 | Calcitriol, CB 1093, EB 1089, KH 1060, MC 903, and MC 1288 | C6 glioma cell line from rat | 24 hours | MTT assay, Northern blot analysis, DNA isolation, and analysis | 10-7 - 10-11 M | KH 1060 had the greatest effect in inducing the death of glioma cells. The effect of MC 1288 and CB 1093 was equal to Calcitriol. EB 1089 was partially less effective than Calcitriol and MC 903 had only a subtle activity. Cell death was caused by the c-myc protooncogene induction. | Effective | Ethanol | Baudet et al. (39) |
| 10 | Calcitriol, Cholecalciferol, All-trans Retinoic Acid (RA) | Human glioblastoma cell lines Hu 70 and Hu 197 | 1 - 8 hours | MTT and Lactic Dehydrogenase Assays, RNA Analysis and Light Microscopy | 0.01 - 100 µM | Glioblastoma cell growth was reduced even at low concentrations of treatments. | Effective | Same cells without any intervention | Magrassi et al. (46) |
| 11 | Calcitriol, 24,25(OH)2D3 | Rat C6 glioma cell line | 6 days | MTT Assay and RNA Analysis; LiCVUrea method, Northern blot analysis | 10-8 - 10-12 M | Cell death occurred even 24 h after the presence of calcitriol. Calcitriol regulated the expression of its own receptors in C6 glioma cells. | Effective | Ethanol | Naveilhan et al. (47) |
| No. | Compound | Animal | Evaluation | Treatment | Main Results | Effectively | Control | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Calcitriol | Mice | Neurosphere formation assay, Tumor xenografts, MRI | Inject 1 μg/kg body weight | Calcitriol reduced the tumor size | Effective | Sesame oil, sterile water | Hu et al. (48) |
| 2 | EM1, calcitriol | Mice | WST-1 colorimetric assay (Roche), FACScan flow cytometry, wound healing assay, cell adhesion assay, matrigel-coated; transwell inserts (millipore), gelatin zymography assay, Western blot, immunofluorescence, RNA extraction, and real-time q PCR, plasma calcium levels in mice and liver and kidney histological analysis, molecular docking | 0.01 - 100 nmoL in vitro (cells); inject 50 µg/kg body weight in vivo (mice) | EM1 and calcitriol treatment reduced cell viability. EM1 had anti-migratory effects and decreased invasive capacity. EM1 bonded to the VDR with greater affinity than calcitriol. EM1 treatment had not induced hypercalcemia or toxicity effects in vivo. | Effective | Isopropanol | Ferronato et al. (18) |
| 3 | Calcitriol, TMZ | Rat | Orthotopic xenograft, MRI | Injection of 0.2 µg/kg/day of calcitriol dissolved in 200 µL of saline solution and also received TMZ as described above. | Combined therapy reduced tumor size and prolonged survival duration in vivo | Effective | dimethyl sulfoxide | Bak et al. (1) |
| 4 | Calcitriol | Rats | Northern blot & RNase protection, immunostaining methods, DNA transection & chloramphenicol acetyltransferase (CAT) assays | Injection of 20 mg/kg body weight | Treatment resulted in a decrease in the level of P75NTR mRNA in the spinal cord. | Effective | Ethanol | Naveilhan et al. (49) |
| 5 | ML-344, calcitriol | Mice | WST-1 colorimetric assay (Roche), cell counting, flow cytometry, wound healing assays, matrigel-coated; transwell inserts (millipore), immunofluorescence, plasma calcium levels in mice, molecular docking studies | 10-11 to 10-7 moL + 100 nmoL extra in vitro (cells); Inject 5 μg/kg body weight in vivo (mice) | Both the ML-344 and calcitriol reduced the cell viability and migration capacity, ML-344 did not hypercalcemic effect in vivo, ML-344 was able to bind to VDR with stronger affinity than calcitriol. | Effective | Isopropanol | Ferronato et al. (16) |
| Author | Study Limitation | Inconsistency | Indirectness | Imprecision | Publication Bias | Dose Effect | Overall Quality |
|---|---|---|---|---|---|---|---|
| Hu et al. (48) | √ | √ | √ | √ | √ | √ | ++++ |
| Ferronato et al. (16) | √ | √ | √ | √ | √ | √ | ++++ |
| Emanuelsson et al. (17) | √ | √ | √ | √ | √ | ×b | +++ |
| Ferronato et al. (18) | √ | √ | √ | √ | √ | ×c | +++ |
| Bak et al. (1) | √ | √ | √ | √ | √ | ×d | +++ |
| Salomon et al. (43) | √ | √ | √ | √ | √ | ×d | +++ |
| Reichrath et al. (49) | √ | √ | ×e | ×f | √ | √ | ++ |
| Diesel et al. (43) | √ | √ | ×e | ×f | √ | √ | ++ |
| Magrassi et al. (46) | √ | √ | √ | Unclearg | √ | √ | +++ |
| Davoust et al. (38) | √ | √ | √ | √ | √ | √ | ++++ |
| Baudet et al. (45) | √ | √ | ×h | ×f | √ | ×d | + |
| Baudet et al. (40) | √ | √ | √ | ×f | √ | ×d | ++ |
| Naveilhan et al. (49) | √ | √ | ×h | ×f | √ | √ | ++ |
| Baudet et al. (39) | √ | √ | √ | √ | √ | √ | ++++ |
| Magrassi et al. (44) | √ | √ | √ | Unclearg | √ | √ | +++ |
| Naveilhan et al. (47) | √ | √ | √ | √ | √ | √ | ++++ |
aGRADE factors: √, no serious limitations; ×, serious limitations; Unclear, unable to rate items based on available information. For overall quality of evidence: +, very low; ++, low; +++, moderate; ++++, high.
bDose-dependency about LDH-release and migration was not implied.
cDose-dependency only about cell viability was implied.
dNo dose-dependency was observed.
eOur pre-defined outcomes comprehensively were not examined.
fNo statistical test was used.
gStatistical analysis for considered results is not shown.
hOutcomes differ from those of primary interest.
| Item | Bak et al. (1) | Ferronato et al. (16) | Ferronato et al. (18) | Naveilhan et al. (47) | Hu et al. (48) |
|---|---|---|---|---|---|
| 1. Was the allocation sequence adequately generated and applied? | U | U | U | U | U |
| 2. Were the groups similar at baseline or were they adjusted for confounders in the analysis? | Y | Y | Y | Y | Y |
| 3. Was the allocation to the different groups adequately concealed? | U | U | U | U | U |
| 4. Were the animals randomly housed during the experiment? | U | Y | Y | U | U |
| 5. Were the caregivers and/or investigators blinded from knowledge which intervention in each animal received during the experiment? | U | U | U | U | U |
| 6. Were animals selected at random for the outcome assessment? | U | U | U | U | U |
| 7. Was the outcome assessor blinded? | U | U | U | U | U |
| 8. Were incomplete outcome data adequately addressed? | U | U | U | U | U |
| 9. Are reports of the study free of selective outcome reporting? | U | U | U | U | U |
| 10. Was the study apparently free of other problems that could result in high risk of bias? | U | U | U | U | U |
Abbreviations: Y, yes; N, no; U, unclear.
3.1. Results of In Vitro Studies
Emanuelsson et al. (17) evaluated the effects of Tacalcitol and Calcipotriol (as vitamin D analogs) on the viability of cells, proliferation, and migratory capacity. T98G cells from human glioblastoma were used in their study. Two analogs and 1α,25(OH)2D3 were added to the culture medium in concentrations from 1 nM to 10 µM. The viability of T98G cells was measured by a colorimetric assay; proliferation was analyzed by manual cell counting, using a hemocytometer and for cell migration “wound healing” assay was taken into account. Both analogs decreased cell viability and/or growth and dose-dependently (P < 0.05 and P < 0.001); they also inhibited proliferation (P < 0.001) and decreased cell migration (P < 0.05).
Salomon et al. (41) investigated VDR expression in human glioma and its relevance to cell viability, migration, and patient survival. The cell lines originating from human GBM (U251, U87MG, and T98G) and human breast adenocarcinoma (T47D) were evaluated in this study. Cell lines were treated with calcitriol (1 µM) or vehicle for 96 hours for cell survival assay. It took 17 hours to check cell migration and 48 hours for immunofluorescence. In comparison with VDR in non-malignant brains, the number of vitamin receptor(s) increased in tumor tissues (P = 0.009). VDR expression was correlated with an improved outcome in patients with GBM (P = 0.046). The results have also shown the modulation of VDR-controlled migration of GBM cells (P = 0.017) and survival (P < 0.001). Treatment with calcitriol decreased the migration rate of T98G cells (P = 0.029).
In an in vitro study on several GBM cell lines, Reichrath et al. (42) assessed the effect of 1,25(OH)2D3 on the key components of the Notch-signaling pathway. Tx3868, Tx3095, U87MG, U118, and U373MG were used in this study. Proliferation assay, Western blotting, Polymerase chain reaction (PCR), and Flow cytometry were applied. Various concentrations of 1,25(OH)2D3, trichostatin A (TSA) /5-azacytidine (5-AZA) or their mixture were put on the medium up to 100 μL volume for 24, 48, and 72 hours. Consequently, some components of Notch-signaling pathways were expressed in some cell lines, but not in some others. Treating GBM cell lines with 1,25(OH)2D3 did not result in significant anti-proliferative effects. The treatment also had no modulatory effect on the gene expression of pivotal components of the Notch-signaling pathway. Combined therapy through 1,25(OH)2D3 plus TSA, or 5-aza did not increase the antiproliferative effects in comparison with TSA or 5-aza alone; it also reduced TSA or 5-aza antiproliferative properties.
Diesel et al. (43) examined the role of vitamin D3 metabolism in glioblastoma multiforme. TX3868 and TX3095 were xenografted on mice. The rest of the GBM cell lines did not undergo xenografting; COS-1 cells and rat GBM cell line C6 were also used in that study. Proliferation assays, nested touchdown reverse transcription-PCR (RT-PCR), real-time RT-PCR, and high-performance TLC were applied. Calcitriol and calcidiol were used in concentrations of 10-12 to 10-6 mol/L and 2.5 × 10-8 mol/L, respectively. The medium culture was restored daily with fresh vitamin D3 metabolites content within 6 days. This study reported the 16 splice variants of CYP27B1 in GBM, which were differentially expressed in cancerous and normal tissues. They expressed the enzymatic activity of endogenous CYP27B1 in GBM cell lines. The proliferative effect of calcitriol on some cell lines from human GBM was reported, but it had no significant effect on the rest of them. In addition, calcidiol had an anti-proliferative effect on 6 out of 9 cell lines and a proliferative effect on the remaining 3 cell lines.
Magrassi et al. (44) examined the effect of cholecalciferol on glioblastoma cells death. Hu197, which is a stable human glioblastoma cell line, was used in this study. In this study, light microscopy, DNA extraction and pulsed-field gel electrophoresis, MTT assay and lipids extraction, and purification were applied; 50 µg of ceramide and 50 µg of cholecalciferol were used separately compared with the control within 6 and 12 hours. After cholecalciferol treatment, intracellular ceramide was increased and induced cell death was observed compared with the control (P < 0.05). Ceramide also resulted in induced cell death in the same cells compared with the control (P < 0.005). These results have shown that the sphingomyelin pathway could be involved in the antitumor activity of vitamin D metabolites.
Davoust et al. (38) reported that the transfection of VDR into a colony of rat glioma cells resistant to 1,25-D3 increased the sensitivity to the cytotoxic effect of this vitamin. C6 cell line from rat glioma was supplied with the C6.9D3Sen and C6.4 D3Res clones. Cell cultures were treated with 10-11 to 10-7 M ethanol 0.1% (vehicle) or 1,25-D3. The duration of treatment was 24 hours. The assays applied in this study included Plasmid construction and transfections, MTT cell viability assay, DNA fragmentation, southern blot, northern blot analysis, and the measurement of 1,25(OH)2D3 specific binding. The treatment of C6.9D3Sen cells for 24 hours with 1,25-D3 at defined concentrations significantly reduced cell numbers compared with control cells (P < 0.01).
Baudet et al. (45), showed antineoplastic effects of 1,25-D3 by examining the genes, which in adherence to the induced cell death by 1,25-D3 were differentially expressed. Rat glioma C6 cell line in the culture medium was treated with 1,25-D3 at 5 × 10-8 M for 24 hours or with vehicle alone (ethanol). Rat glioma C6.9 involved in the cell death program was examined in terms of gene expression through the differential analysis of a rat brain cDNA library, using probes containing control and 1,25-D3-treated cells. Their methods included the following assays: cDNA library and high-density filters, hybridization and differential screening analysis, DNA sequencing and computer analysis, Northern blot analysis and cDNA probing, and the evaluation of the length of inserts. Consequently, 61 differentially expressed cDNAs were reported. Among them, 1,25-D3 was able to down-regulate the expression of peripheral myelin protein 22 (PMP22/gas3), secreted protein acidic and rich in cysteine/osteonectin (SPARC/ON) MAP1C/dynein heavy chain, S100β and aldolase C genes, and was able to up-regulate cysteine-rich protein, matrix gamma carboxyglutamic acid protein (MGP), β-Tubulin, and mortalin. These changes caused the programmed cell death induced by 1,25-D3.
Baudet et al. (40) investigated the mechanisms leading to glioma cell death induced by 1,25(OH)D3 in the rat. C6.9 and C6.2 clones were used. Cells were exposed to 10-7 M 1,25(OH)2D3, or vehicle for 24 hours. MTT assay, DNA isolation and analysis, RNA isolation and northern blot, DNA flow cytometry, and electron microscopy were applied. The findings of this study showed that cell death induced by 1,25(OH),D3 depends on the synthesis of certain proteins along with some genes expression, including c-myc, p53, and gadd45. After the addition of 1,25(OH)2D3, the expression of two genes encoding the interleukin-6 and vaso-endothelial growth factor were also increased.
Baudet et al. (39), in an in vitro study, evaluated the effects of different vitamin D3 analogs with the lowest calcemic effect in vivo on the growth of glioma cells. C6 glioma cell line from rat was used. Various concentrations of 1,25(OH)2D3, its analogs, or ethanol (as a vehicle) were put on the medium culture for 24 hours. The analogs used in this study included CB 1093, EB 1089, KH 1060, MC 903, and MC 1288. The most effective doses were approximately 10-9 M and 10-10 M for l,25(OH)2D3 and KH 1060, respectively (P < 0.001). MTT assay, northern blot analysis, DNA isolation, and analysis were applied in the study. The compound KH 1060 had the greatest effect in inducing the death of glioma cells (P < 0.001). The effect of MC 1288 and CB 1093 was equal to 1,25(OH)2D3. The effectiveness of EB 1089 was partially less than 1,25(OH)2D3. MC 903 had only a subtle activity on C6.9 cells. DNA fragmentation and induction of the c-myc protooncogene were also accompanied by this cytotoxicity effect.
Magrassi et al. (46), in an in vitro study, showed the effects of vitamin D and its metabolites along with retinoic acid on human glioblastoma cells. Hu 70 and Hu 197 were used in the study. 1,25(OH)2D3, Cholecalciferol, and all-trans retinoic acid (RA) were purchased. MTT and Lactic Dehydrogenase assays, RNA analysis, and light microscopy were applied in their study. Both substances reduced glioblastoma cell growth at a concentration of more than 5 µM. This was significantly inhibited after the dilution of 25-dihydroxy vitamin D3 and combination with 1 μM of all trans-retinoic acid even after adding vitamin D at the concentration range of nanomolar.
Naveilhan et al. (47) conducted a study aimed at in vitro investigating the effects of 1,25(OH)2D3 on cell lines from glioma. Cell culture was provided through the rat C6 glioma cell line. The 1,25(OH)2D3 and 24,25(OH)2D3 were used in the study. MTT assay and RNA analysis were applied. 1,25(OH)2D3 remained in the medium culture for 3 days, the cytotoxic effects were mediated by 1,25(OH)2D3, which were detected in approximately 10-8 M of this vitamin (P < 0.001 compared to control). However, the continuous stay of 1,25(OH)2D3 in the medium was not necessary because cell death happened when 1,25(OH)2D3 presented only for 24 hours and, then, the cells were cultured without the 1,25(OH)2D3. Furthermore, 1,25(OH)2D3 could regulate its own receptors in C6 cells.
3.2. Results of In Vivo Studies
Hu et al. (48) conducted a study on human glioma cell lines (U87MG and T98G), U251 cells, and normal cells originated from human astrocytes. They used 10 and 100 nmoL 1,25(OH)2D3 (calcitriol) to treat stem cell-like glioma cells (SLCs) for 4, 8, 12, 24, and 48 hours in the circumstances with pH 7.4 and 6.8. The expression of stemness markers, self-renewal ability of SLCs, and pre-treated mitochondrial oxygen consumption rates of SLCs were measured. Calcitriol could suppress the stemness of SLCs to some extent. Furthermore, self-renewal ability was repressed in pH 7.4 compared with pH 6.8 (P < 0.01) and in two groups of calcitriol compared with the control (P < 0.001). The basal and maximal respirations of these cells were retarded with calcitriol treatment (P < 0.01). In addition, according to the photos of magnetic resonance imaging, the in vivo treatment of 1 μg/kg calcitriol on mice reduced the tumor size. These results suggested that calcitriol could be a novel and safe treatment for malignant glioma.
Ferronato et al. (18) evaluated the effects of an analog of calcitriol called EM1 on GBM cells in the in vitro, in silico, and in vivo manner. Human U251 and T98G GBM, in addition to human primary astrocytes, were used in their study. With the range of concentrations from 0.01 to 100 nM of EM1, calcitriol or vehicle was used to cell treat for 120 hours. EM1 and calcitriol treatment reduced cell viability compared with the vehicle (P < 0.001). EM1 also had anti-migratory effects on GBM cells (P < 0.01) and decreased their invasive capacity (P < 0.001). In silico EM1 had a higher affinity for the VDR than calcitriol. In the end, mice were injected with 50 µg/kg from EM1 within 21 days without any raise in the serum level of calcium or toxicity.
Bak et al. (1) conducted a study with the aim of examining the antitumor efficacy of combination therapy with vitamin D and temozolomide in glioblastoma. The glioblastoma cell (C6) origin from rat was used. The treatment was taken for 24 hours with dimethyl sulfoxide (DMSO) alone (as a control group), 1 mM TMZ, 100 nM Vitamin D, or a mixture from 1 mM TMZ plus 100 nM Vitamin D. Moreover, 24 male 2-month-old Sprague-Dawley rats with 250 g to 300 g body weight were examined in this study. The assays demonstrated that the treatment of C6 cell line with TMZ and vitamin D resulted in significant increased antitumor effects compared with each of them alone (P < 0.001). This is also true for autophagy (P < 0.001). Furthermore, combined therapy with TMZ and vitamin D significantly restrained tumor advancement and induced a longer lifespan compared with TMZ alone in the rat (P < 0.001).
In an in vitro and in vivo study, Naveilhan et al. (49) reported the vitamin D3 and its metabolite's effects on the regulation of low-affinity neurotrophin receptor. They also revealed whether 1,25-(OH)2D3 could regulate their expression in primary astrocytes and in C6 cells that undergo the program of cell death caused by 1,25-(OH)2D3. C6 cell lines and primary cultures of astrocytes from rats were used. The concentration of 10-8 M 1,25-(OH)2D3 or vehicle was used for 24 hours. To run in vivo section of the study, female 15-day-old rats (Sprague-Dawley) were injected with 20 mg/kg 1,25-(OH)2D3 or vehicle alone for 24 hours. Northern blot and RNase protection, immunostaining methods and DNA transection, and chloramphenicol acetyltransferase (CAT) assays were applied. Even low amounts of 1,25-(OH)2D3 and its metabolites could increase the gene expression and protein levels of low-affinity neurotrophin receptor (P75NTR) in C6 cell lines. They also induced the expression of its own receptor in a similar manner to the P75NTR mRNA. Even the high concentrations of 1,25-(OH)2D3 in the primary cultures of astrocytes did not result in the regulation of P75NTR. In vivo 1,25-(OH)2D3 has decreased the expression of the P75NTR gene in the spinal cord in healthy samples, but it did not affect its expression in dorsal root ganglion or sciatic nerve. In this study, it was revealed that based on the 1,25-(OH)2D3 activity on C6 cell lines, it could be a promising approach for describing the act of P75NTR in cell death.
Ferronato et al. (16) examined the effect of a synthetic novel derivative of vitamin D3 called ML-344 on in vitro antitumor activity and in vivo calcemic effects. They also had the computational studies to examine its affinity for VDR through in silico assays. They focused on various types of cancer cell lines, including glioblastoma cells (U251, GL26, and T98G). To measure cell viability, cells were cultured in medium with 10-11 to 10-7 M of calcitriol, ML-344, and vehicle for 120 hours. In addition, to evaluate the migration of cells through “wound healing” assays, the extra 100 nM of ML-344 or calcitriol was added to the medium. To check the calcemic effect of ML-344, they continued their investigation by injecting a 5 μg/kg body weight of the ML-344 in mice with ML-344, calcitriol or vehicle. Cell viability and migration capacity were retarded by both ML-344 (P < 0.001) and calcitriol treatment (P < 0.01) compared with the control. In addition, despite calcitriol, ML-344 did not cause hypercalcemic effects in CF1 mice (P < 0.05 and P < 0.001). ML-344 was able to bind to VDR with a stronger affinity than calcitriol.
4. Conclusions
This systematic review has provided in vitro and in vivo evidence to investigate the effects of vitamin D3 and its analogs on malignant gliomas. The majority of our findings suggest that vitamin D3 and its analogs, compared with the control, induced cell death and apoptosis and reduced cell growth, invasion, and migratory capacity in gliomas cell culture. Some in vivo studies indicate that calcitriol administration was accompanied by a reduction in the tumor size, and vitamin D3 analogs were able to show the same effects without the hypercalcemic effect in vivo. This systematic review can be a platform for researchers to sum up the antitumor effects of vitamin D3 and consider vitamin D3 and its analogs as a safe curable supplement along with routine therapies for malignant gliomas. However, there is a necessity for high-quality studies in the future to better understand the mechanisms and to investigate the effects of this vitamin on health status and clinical outcomes in patients with malignant gliomas.