1. Background
Prostate cancer is the second most common malignancy among the male population worldwide (1) and it is also the second leading cause of cancer related mortality in American men (2).
Radiotherapy alone or along with surgery and hormontherapy are the main treatments in patients with prostate cancer (3). Because the prostate is located close to the rectum and bladder, these organs are at risk for developing treatment-related complications (4). The majority of the acute GI and GU toxicities caused by EBRT in prostate cancer patients relate to mucosal loss and inflammation in the rectum, bladder neck, within the prostate and prostatic urethra (5).
COX-2, an inducible isozyme, is dramatically overexpressedsin responses to cytokines, mitogens, growth factors, and various other stimuli that are correlated with a range of processes including inflammation (6). COX-2 has been reported to interact with angiogenic, apoptotic, proliferative, invasive, metastatic and other pathways involved in cancer progression (7-9). Cox-2 is also correlated with poor differentiation, increased nodal involvement, increased tumor size and distant disease, and decreased overall survival in a variety of cancers (10-12). Many studies have found COX-2 over expression in prostate cancer (13, 14) and its level of expression associated with Gleason score and cancer progression (15).
Regular use of non steroidal anti-inflammatory drugs (NSAIDs) may diminish the risk of certain types of human cancer (16, 17). The importance of these drugs in cancer chemoprevention has been supported by many studies that show decreased risk of colorectal cancer (18, 19) and breast cancer (20), in those individuals who regularly consume aspirin or other NSAIDs.
Conventional NSAIDs blocked the activity of both COX-1 and COX-2. Unfortunately, NSAID usage is correlated with gastrointestinal toxicity and changed platelet function (21). Selective cyclooxygenase (COX)-2 inhibitors containing celecoxib have been developed as safer alternatives to conventional COX inhibitors (22).
The ability of such medications to selectively inhibit COX-2 (against traditional nonsteroidal anti-inflammatory medications that blocked the activity of both forms of COX) permits for the specific anti-inflammatory benefits without the related toxicity (gastrointestinal, renal, and bleeding), which is taken from COX-1 Inhibition (23). Compatible to other selective COX-2 inhibitors, celecoxib shows diminished risk of upper gastrointestinal ulcer complications (24). Furthermore, selective inhibitor of COX-2 greatly enhanced tumor radioresponse and protects normal cells during irradiation (25).
Selective COX-2 inhibitors blocked growth of human prostate cancer cells in vitro and in vivo by inducing apoptosis and inhibiting cell proliferation and angiogenesis (26-29). Nowadays, celecoxib is a choice for clinical trials because of the preferable anticancer and cardiovascular safety profile (30, 31).
2. Objectives
The purpose of this study was to assess the effect of celecoxib against radiation-induced acute bowel and urinary toxicities in patients treated with radiotherapy for prostate cancer using radiation therapy oncology group (RTOG) acute radiation morbidity grading criteria.
3. Methods
From 2015 to 2016, all patients recently diagnosed with localized prostate cancer at the radiotherapy department of media center in Hamadan, Iran were evaluated for eligibility for participation in this study. Referred patients to local-curative radiotherapy with external beam radiotherapy (EBRT) were invited to take part in the project.
3.1. Inclusion and Exclusion Criteria
Histologically confirmed prostate cancer cases were candidates for definitive or postoperative external beam radiotherapy and Karnofsky performance score of more than 80 % were included after acquiring informed consent. Additional inclusion criteria were normal levels of hemoglobin, leukocytes, platelets, creatinine, urea, alkaline phosphatase (AP), aspartat-aminotransferase (AST), alanin-aminotransferase (ALT) and bilirubine in the patients.
Exclusion criteria included prior treatment with radiotherapy or chemotherapy, clinical and radioghraphic evidence of distant metastasis before or during the trial, inability to receive celecoxib (e.g. gastric ulcer, allergy to COX-2 inhibitors), simultaneous participation in another clinical trial, which would need approval upon entry to this trial, receiving any other NSAIDs, presence of severe and uncontrolled cardiovascular, kidney, liver, inflammatory intestinal disease, or coagulation disorders.
3.2. Ethical Considerations
All patients provided their written informed consent prior to participation. This trial was registered at http://www.irct.ir (IRCT2016020626401N1) and approved by the ethics committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (approval number: SBMU.REC.1393.731). The data is retained regardless of the patient’s name.
3.3. Randomization
Participants were randomly allocated to either the celecoxib group (CG, n = 20) or a placebo group (PG, n = 20). Randomization was carried out by administrative personnel outside the trial in a double-blind fashion. All participants and researcher were blinded to random group assignment.
3.4. Intervention
All participants were treated orally with celecoxib 200 mg or placebo twice per day in an open label during the entire series of radiation therapy. The intake of celecoxib or placebo was started on the first day of radiotherapy and continued without interruption until completion of their radiotherapy. The first celecoxib or placebo was administered 3 hours before each radiotherapy fraction and the second, 12 hours after the first consumption. Medication was continued until progression of the diseases or unacceptable toxicity.
3.5. Radiotherapy Techniques
All patients underwent CT scanning with 3 mm thickness of the pelvic in order to confirm prostate location relative to the treatment fields. The preparation for CT scan comprises dietary guidelines, the administration to a mini enema, use of anti-gas tablets to ensure an empty rectum, and at least 200 Ml of water 20 - 30 minute prior CT to achieve a full bladder. The planning CT scan was done with the patient in supine position with feet rests, from L4 - L5 junction to 10 cm caudal to the ischial tuberosities. Clinical target volume (CTV) contained the prostate gland or prostate gland together with the seminal vesicles. 1 Cm margin added environs the CTV for defining the planning target volume (PTV), except at the boundary between the anterior rectal wall and the prostate where a 0.6-cm margin was used.
For all patients a conventional 4-field technique in supine position with daily fractions of 1.8 - 2 Gy (5 times per week) was used. Whole-pelvic radiotherapy followed by a cone down to the prostate and seminal vesicles was exerted to 13 patients in celecoxib group and 14 patients of the placebo group. A total of 12 patients (6 patients in each group) received radiation to the prostate and seminal vesicles alone. All patients received neo adjuvant hormone therapy.
3.6. Clinical Evaluation
Prior to inclusion into the trial, all the participants were asked about any history of allergic reaction to no steroidal anti-inflammatory drugs, celecoxib intolerance, gastrointestinal bleeding, gastrointestinal ulcer, hepatic and/ or renal dysfunction, and/or insufficiency.
The pre-therapeutic staging examinations included the initial PSA value, biopsy with histological confirmation, and statement of the Gleason score, rectal examination, computed tomography (CT), in order to assess the lymph nodes and whole body bone scan was performed.
Stages were assigned according to the 7th edition of the American joint committee on cancer (AJCC) TNM 2010 staging system. The patients’ functional status were evaluated conforming to the Karnofsky performance status.
Before starting therapy, in 4 weeks of the therapy and 1 month after completion of the treatment, blood samples were taken. The measurements contained complete blood count, coagulation parameters and creatinine level, serum urea, AP, AST, ALT, and total bilirubine.
Acute bowel and urinary toxicities, according to RTOG criteria were recorded pre-treatment, were at least once weekly during radiotherapy and once a month after the end of the treatment. Acute toxicity was present when one of GI or GU symptoms occurred within 90 days after the start of the treatment (32).
3.7. Statistical Analysis
Statistical analyses were performed using STATA software version 12. A P value was a two-sided test and the level of statistical significance was set at P < 0.05. Patients’ age and percentage of rectum and bladder volume at 60 Gy were summarized by mean and standard deviation values. The Shapiro-Wilk test was used to find normal distribute between the two groups in terms of the characteristics of patients and treatment-related parameters. The Chi-squared (χ2) test or Fisher’s exact were used to find a significant difference in the number of patients with acute toxicity between celecoxib and placebo group. A logistic regression analysis was used to adjust for the effects such as age and Gleason score and tumor stage.
4. Results
Thirty-nine patients (39/40) fully completed the treatment, and according to protocol, took all their supplements. We used face to face interview for completion of questionnaires.
The patients tolerated celecoxib well, no cardiovascular, gastric, renal, hepatic, or bone marrow side effects of celecoxib occurred. No patient experienced delays or revocation of treatment due to toxicity except for one patient of celecoxib group that relinquished treatment continuation after 21 fractions due to diarrhea. All patients received neoadjuvant hormone therapy. The baseline characteristics for 39 patients and treatment-pertinent parameters are shown in Table 1. There was a normal distribution between the two groups with respect to characteristics of patients and treatment-related parameters.
| Characteristics | Placebo Group B (n = 20) | Celecoxib Group A (n = 19) |
|---|---|---|
| Age | 69.85 ± 10.17 | 70.57 ± 9.06 |
| Baseline PSA, ng/ML | ||
| 0 - 9.9 | 9 (45) | 8 (42) |
| 10 - 19.9 | 6 (30) | 9( 47) |
| 20+ | 5 (25) | 2 (11) |
| Gleason sum at diagnosis | ||
| 2 - 6 | 4 (20) | 6 (32) |
| 7 | 5 (25) | 7 (37) |
| 8 - 10 | 11 (55) | 6 (32) |
| Tumor stage | ||
| T2 | 10 (50) | 9 (47) |
| T3 | 8 (40) | 9 (47) |
| T4 | 2 (10) | 1 (5) |
| Largest field treated | ||
| Pelvis | 14 (70) | 13 (68) |
| Prostate + seminal vesicles | 6 (30) | 6 (32) |
| Postoperative RT | 9 (45) | 8 (42) |
| V60 (Rectum), Mean % (SD) | 33.35 (9.03) | 38.05 (10.9) |
| V60, (Bladder), Mean % (SD) | 39.4 (11.52) | 41.68 (13.25) |
Patient Chracteristics and Treatment-Pertinent Parametersa
No gastrointestinal (GI) or genitourinary (GU) acute toxicity grade 4 occurred.
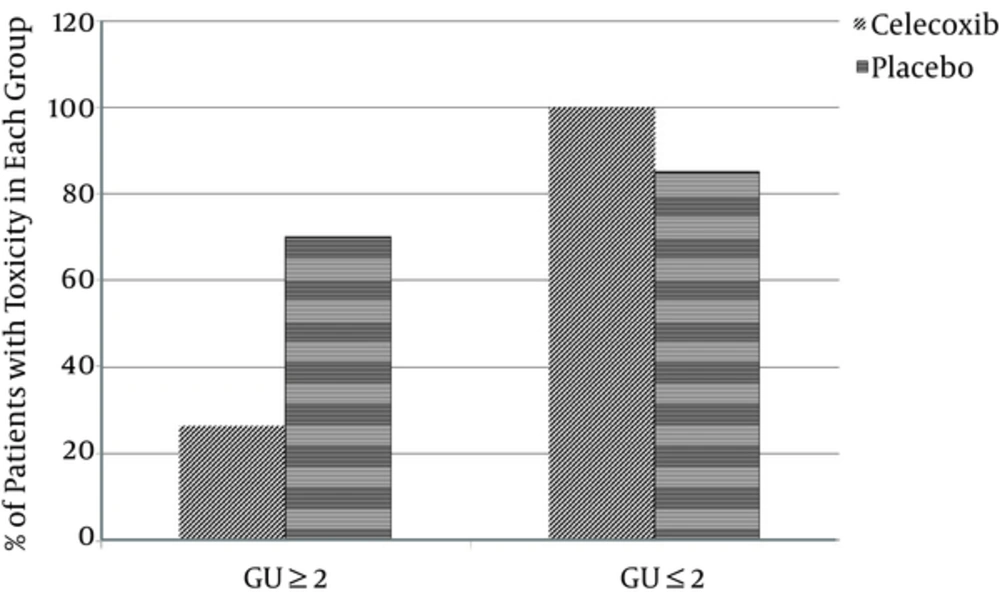
In celecoxib group in terms a GU toxicity 2/19 patients showed grade 0. 12/19 patients showed grade 1 and 5/19 patients showed grade 2. In placebo group in 1/20 patients, we observed a GU acute toxicity grade 0, in 5/20 grade 1, in 11/20 grade 2 and in 3/20 an acute toxicity grade 3(as shown in Figure 1).
Acute GI toxicity in the celecoxib group during treatment was common with grade 0 in 1/19 patients, grade 1 in 10/19 patients and grade 2 in 8/19 patients. Acute GI toxicity in the placebo group during treatment was common with grade 0 in 2/20 patients, grade 1 in 8/20 patients, grade 2 in 9/20 patients and grade 3 in 1/20 patients.
One month after the treatment, most GU and GI symptoms had already retreated. Celecoxib group experienced a lesser extent of toxicity in terms of the GU toxicity especially dysuria, frequency, and urgency compared with the placebo group. However, patients in the celecoxib group experienced a lesser extent of toxicity in terms of the GI toxicity especially diarrhea, rectal and abdominal pain. Compared with placebo group, no significant difference was observed between two groups.
A significant reduction in GU ≥ 2 toxicity was observed in the celecoxib group (P = 0. 006). No significant difference was noted in terms of GI ≥ 2 toxicity between two groups of celecoxib and placebo (P = 0.62) (Table 2) but the celecoxib group was experiencing 6% lower GI ≥ 2 toxicities compared with the placebo group (OR = 0.94, 0.95CI: 0.24 - 3.74, P = 0.93).
| Toxicity | Placebo (n = 20) | Celecoxib (n = 19) | P Value |
|---|---|---|---|
| GU ≥ grade2 | 14 (70) | 5 (26.32) | 0.006 |
| GU ≤ grade1 | 17 (85) | 19 (100) | 0.07 |
| GI ≥ grade2 | 10 (50) | 8 (42) | 0.62 |
| GI ≤ grade1 | 19 (95) | 19 (100) | 0.32 |
Acute GI and GU Toxicities and Differences Between the Two Groupsa
Odds of GU ≥ 2 toxicities in celecoxib were 86% lower than the placebo group (OR: 0.14, 0.95CI: 0.03 - 0.62, P = 0.01) (Table 3).
| Genitourinary ≥ 2 | Gastrointestinal ≥ 2 | |||||
|---|---|---|---|---|---|---|
| OR | P Value | %95 CI | OR | P Value | %95 CI | |
| Celecoxiba | 0.15 | 0.009 | 0.03 - 0.62 | 0.72 | 0.62 | 0.21 - 2.6 |
| Celecoxibb | 0.14 | 0.01 | 0.03 - 0.62 | 0.94 | 0.93 | 0.24 - 3.74 |
| Age | 0.95 | 0.24 | 0.88 - 1.03 | 1.03 | 0.44 | 0.96 - 1.11 |
| Gleason score | 0.93 | 0.79 | 0.54 - 1.6 | 1.55 | 0.11 | 0.9 - 2.66 |
| Tumor Stage | 0.84 | 0.77 | 0.25 - 2.74 | 0.56 | 0.31 | 0.18 - 1.73 |
Logistic Regression Analyses
5. Discussion
Several reports have demonstrated that the COX-2/PGE2 signaling pathway plays an important role in the progression of malignant tumors (7-9, 33). Furthermore, COX-2 has been shown to affect carcinogenesis, tumor proliferation, metastasis, angiogenesis, and tumor resistance to anti-cancer drugs (34).
Celecoxib may play a role in the treatment of cancers by affecting cell proliferation, promotion of apoptosis, and cell communication and integrity (35-37). Moreover, celecoxib exerts an anticancer effect by sensitizing cancer cells to apoptosis by inhibiting angio-genesis, the up-regulation of Bax expression, the down-regulation of Bcl-2, cyclooxygenase-2, PAkt, and carbonic anhydrase, and through the eventual radiosensitization which promotes tumor tissue apoptosis (38, 39). The fundamental mechanism of radiosensitization by celecoxib may be relevant to regulating IR-induced G2/M arrest (40). Shin et al. indicated that the radiation-enhancing effects associated with celecoxib occur in cancer cells in a COX-2 expression-dependent manner and do not seem to originate from reduced PGE2 generation. Celecoxib may attenuate radiation-induced G2-M arrest in COX-2-overexpressing cells, which may allow the arrested cells to enter mitosis and die after radiation. In contrast, in COX-2, low-expressing cells enhanced radiation-induced G2-M arrest (41).
The current study focused on prostate cancer to determine whether celecoxib with its radiosensitive effects affects normal cells in the bladder and rectum. For this purpose, acute GI and GU toxicities in patients in a celecoxib group and a placebo group were assessed and compared.
Despite the higher dose delivered to the rectum and bladder in the celecoxib group compared to the one in the placebo group, a significant reduction in GU toxicity was noted in the celecoxib group. Patients in the celecoxib group also experienced lesser GI toxicity compared with the placebo group, but no significant difference between the two groups was observed.
The mechanisms responsible for the radioprotective effects of celecoxib on urinary and intestinal tracts were not specially examined in this investigation. However, the radioprotective effects of celecoxib could come about through decreasing COX-2 levels. COX-2 plays a critical role in the convergence of various upstream pathways of inflammation, including IL1 and IL6 signaling (42).
Celecoxib decreases pro-inflammatory cytokines (43) and prevents the activation of TNF-α-induced NF-Kb (44). Javle et al. showed celecoxib ameliorated diarrhea and weight loss in rat models (45). Consequently, the ability of celecoxib to reduce toxicity and its safe administration, accessibility, and oral administration capability introduce it as a proper radioprotector against acute radiation-induced toxicity.
In various clinical trials amifostine, famotidine, and supplemental curcumin have been known as radioprotectors against related radiation toxicity.
A study by Razzaghdoust et al. that included 36 prostate cancer patients indicated that famotidine significantly reduced rectal toxicity. In this trial, famotidine was also well tolerated. This study suggested famotidine as a proper radioprotector for rectal mucosa (46).
A phase II trial by Dunst et al. included 30 patients with stage I/II rectal cancer who were treated with adjuvant chemoradiation. The researchers demonstrated that amifostine significantly reduced acute skin and rectal toxicity, and they reported several amifostine-pertinent toxicities, included hypotension (53% grade I, 7% gradeII) and nausea (47% grade I, 13% gradeII) (47).
In a phase II study, Koukourakis et al. evaluated 40 patients with pelvic tumors. Their results indicated that subcutaneous amifostine in 85% of patients was well tolerated, and a significant reduction in acute rectal and perineal skin and bladder toxicity was observed in the amifostine group versus the control group. In this study, several patients required an interruption in amifostine administration because of drug-related toxicity (48). Of course, amifostine has limitations such as the presence of side effects, the required monitoring of blood pressure, and the need to be administered in high doses.
The results of a study by Hejazi et al. indicated that supplemental curcumin as a radioprotector can reduce the severity of radiotherapy-related urinary symptoms in patients with prostate cancer (49).
Several clinical trials indicated that celecoxib combined with radiation can be safely administered and is well tolerated.
In a clinical phase I trial, the acute toxicity of celecoxib administered during percutaneous radiotherapy was evaluated in 22 patients with localized prostate cancer. All patients received oral celecoxib 400 mg twice daily. In the second week of treatment, 2 of the 22 patients showed a general exanthema with pruritus (drug allergic reactions); medication was stopped and the complications were resolved. In the results, no grade 3 or grade 4 GI or GU toxicity was seen. GI acute toxicity grades 1 and 2 was manifested in 85% and 10% of 20 patients, respectively. 80% of patients showed grade 1 GU toxicity, and 10% had grade 2 complications. Compared with the published data, the combination of radiotherapy for prostate cancer and simultaneously administered, highest FDA-approved dose of celecoxib did not correlate with an increased level of toxicity (50), which is in agreement with the current study. Unfortunately, in this study the toxicities in the celecoxib group were not compared with those of the placebo group.
A trial by Johnny Kao et al. demonstrated that the concurrent administration of erlotinib, celecoxib at escalated doses (200,400,600 mg twice daily), and reirradiation for a population of patients with recurrent head and neck cancer is an active regimen and safe administration (51).
The results of another study in patients with biochemical progression following definitive radiation therapy or radical prostatectomy indicated that celecoxib (400 mg twice daily) may affect the decline or stabilization of PSA levels, and therefore help delay or prevent disease development. Follow-up PSA levels to assess efficacy were obtained at 3, 6, 12, and18 months after initiation of treatment and subsequently every 6 month thereafter. In this study, no other cardiovascular or further side effects of celecoxib were encountered (52). The follow-up PSA levels of patients in the celecoxib group and the placebo group were not compared in the current study because of its short duration follow up.
The current results and those of a multitude of studies indicate that celecoxib is safe to administer, but clinical trials, especially those with complex regimes, do not allow an incautious use of coxibes. In this regard, Gaffney et al. indicated that celecoxib at 400 mg twice daily with concurrent pelvic radiotherapy, cisplatin, and 5-flurouracil for patients with locally advanced cervical cancer have a major GI toxicity in ~ 50% of the treated patients (53). Similarly, another study on patients with pancreatic cancer indicated that celecoxib added to chemoradiation with gemcitabine revealed more toxicity (54).
The combination of celecoxib with radiotherapy is well tolerated and significantly decreased acute urinary toxicities in patients with prostate cancer that we recommended celecoxib as a suitable radioprotector for reduced acute toxicities related radiotherapy for patients with prostate cancer.
There are some limitations in this study, including short duration of the follow up and small sample size. Larger clinical trials with different doses of celecoxib are desirable to further confirm radioprotective effects in other organs. Clinical trials testing celecoxib to assess quality of life in patients with prostate cancer, and clinical trials to assess the radioprotective effect celecoxib concurrent famotidine in patients with prostate cancer.