1. Context
Breast cancer is the most common cancer among women, worldwide (1) that comprises 16% of all female cancers (2). Around 235,303 new cases of breast cancer, and about 40,430 deaths per year have been reported in the United States (3). In developed countries, this cancer comprises approximately 10% of all cancers and 23% of women cancers (4). The incidence of breast cancer is increasing in developing countries with a rate of 3% to 4% (5) and the breast cancer is appeared around 1 decade earlier than that in developed countries. Early detection of breast cancer reduces the mortality rates and improves patient prognosis (1).
The etiology of breast cancer is not well known, but a family history of the disease, genetic background, high level of estrogen, reproduction history, age at menarche and menopause, dietary habit, life style, cigarette smoking, age, and alcohol consumption are some risk factors of this cancer (6, 7). Population studies suggest that genetic factors, including gene polymorphisms and the presence of mutations might be strong risk factors that influence the individual differences in susceptibility to breast cancer (8).
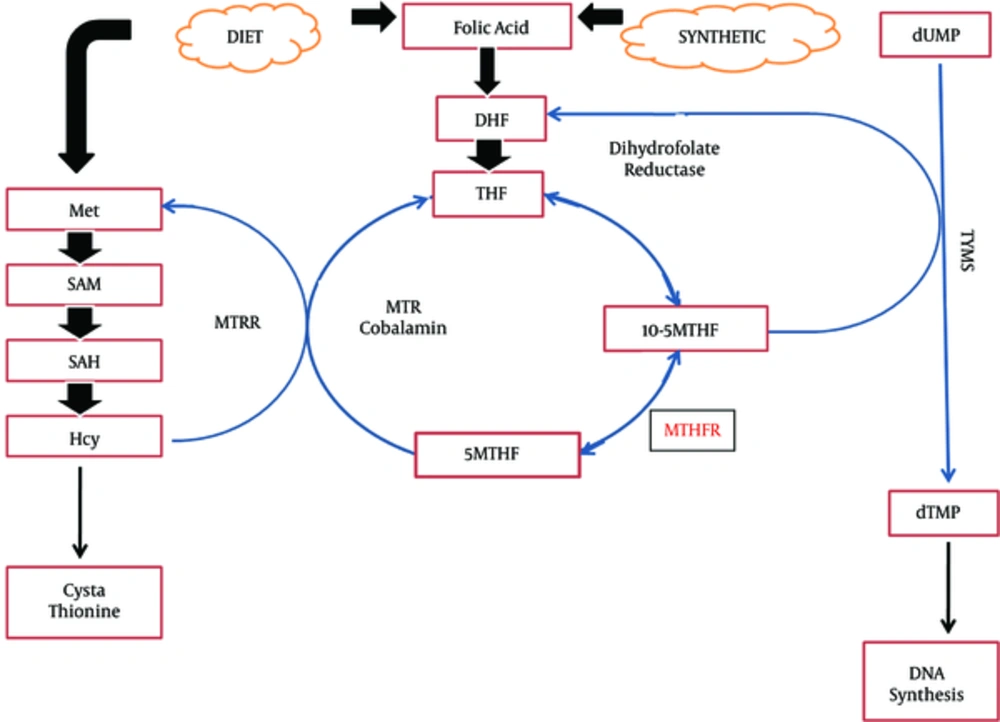
Due to the critical role of one-carbon metabolism in the synthesis and methylation of DNA and cross-talk between genetic and epigenetic processes, interruption of one-carbon metabolism may play important role in the etiology of breast cancer (9). Folate, a water soluble necessary vitamin, is a key element in the one-carbon group metabolism that is required for DNA synthesis, repair, and methylation (10). In Figure 1, the pathway of one-carbon metabolism is demonstrated. Folate deficiency has been associated with some cancers including breast cancer (11). An association between folic acid deficiency and increased risk of cancer through DNA damage by uracil misincorporation into DNA, DNA hypomethylation/dysmethylation, and increased cytosine deamination in the sites of DNA methylation has been suggested (10, 12). One-carbon metabolism pathway plays a key role in genome integrity, DNA methylation, and gene expression. So, any aberration in this pathway might be associated with the risk of breast cancer and its phenotype (13).
One-Carbon Metabolism Pathway; DHF, dihydrofolate; THF, tetrahydrofolate; 5-10 MTHF, 5-10 methylenetetrahydrofolate; 5MTHF, methyltetrahydrofolate; 5MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; HCY, homocysteine; TYMS, thymidylate synthase; dTMP, deoxythymidine monophosphate.
Single nucleotide polymorphisms (SNPs) in folate related genes are suitable candidates for investigating the role of these variants in the susceptibility to breast cancer (14). Variants of methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C are good candidates to study the role of genetic variants of folate metabolizing enzymes in the risk of breast cancer. The present review looks at the role of MTHFR C677T and MTHFR A1298C variants, synergism between these gene variants as well as the effect of folate intake in susceptibility to breast cancer in various populations.
2. Evidence Acquisition
Relevant English language studies were searched, using the search engines such as MEDLINE/PubMed, EMBASE, and Scopus with key words relevant to MTHFR C677T polymorphism, MTHFR A1298C polymorphism, folate intake, B vitamins, and breast cancer from 2005 to 2016.
3. Results
3.1. MTHFR C677T Variants in Breast Cancer
MTHFR is a key enzyme that catalyzes the remethylation of homocysteine to methionine through conversion of 5, 10-methylenetetrahydrofolate (5, 10-MTHF) to 5-methyltrahydrofolate (5-MTHF) (15). This enzyme plays a key role in the equilibrium of methyl group sources for DNA methylation and DNA synthesis (16). DNA methylation is an epigenetic mechanism that controls biologic processes (17).
The gene of MTHFR is located on chromosome 1p36.3 (16) and has several polymorphisms, 2 most common of which are MTHFR C677T and MTHFR A1298C. The point mutation of C to T at nucleotide 677 of the MTHFR gene (Ala222Val, rs1801133) causes an alanine to valine change at position 222 of the polypeptide that result in a thermolabile enzyme with reduced catalytic activity (18). Each copy of the 677T allele results in a 35% decreased MTHFR activity. In homozygous genotype of MTHFR 677TT, the MTHFR enzyme has 30% full activity. However, in the presence of heterozygous genotype of MTHFR 677CT, the activity of enzyme is 65 % (19).
There are controversial reports related to the role of MTHFR C677T variants in susceptibility to breast cancer in various populations (Table 1). Among Americans, there are reports of association between MTHFR C677T with the risk of breast cancer (9, 20). However, in a study among both pre- and post-menopausal American women, the MTHFR C677T polymorphism was not associated with the risk of breast cancer (21). Among a population from England, an association was found between the increased risk of breast cancer with MTHFR C677T polymorphism in women under 40 years (22). In Swedish women older than 55 years, the MTHFR 677T allele was associated with higher risk of breast cancer (23). The study suggested an interaction between the MTHFR 677T allele with the age and the importance of DNA methylation in the development of post-menopausal breast cancer (23). In a south-eastern European population, MTHFR C677T was associated with the risk of breast cancer. The menopausal status did not affect the association between the MTHFR gene polymorphisms and breast cancer risk (24). Among Mexican women, the MTHFR 677TT genotype was associated with breast cancer susceptibility (25). Also, among Brazilian women, a significant association was found for women upper the age of 50 years between the risk of breast cancer development and this polymorphism (26). The higher risk of breast cancer in post-menopausal women than the premenopausal women could be due to different expression of estrogen receptor in the first group and the possible estrogen receptor gene misregulation in the presence of MTHFR 677TT genotype (27). In contrast, in a large sample of Canadian women, the MTHFR C677T was not a risk factor for breast cancer (28). Likewise, among women from Spain (29), Germany (30), and West Siberian Region of Russia (31), the MTHFR C677T was not a risk factor for breast cancer.
| Population | Association (+/-) | Interpretation | Reference |
|---|---|---|---|
| American, European, Mexican | + | Association with the breast cancer risk | Chen et al., Xu et al., Papandreou et al., Ramos-Silva et al. |
| British | + | Association with premenopausal breast cancer | Campbell et al. |
| Swedish, Brazilian | + | Association with postmenopausal breast cancer | Ericson et al., de Cassia Carvalho Barbosa et al. |
| American, Canadian, Spanish, German, Russian | - | Lack of association with the risk of breast cancer | Platek et al., Kotsopoulos et al., Henriquez-Hernandez et al., Justenhoven et al., Vainer et al. |
| Asians | |||
| Chinese, Iranian, Jordanian, Turkish, Kazakh, Indians | + | Association with the breast cancer risk | Weiwei et al., Jiang-hua et al., He et al., Hosseini et al., Awwad et al., Ozen et al., Akilzhanova et al., Mohammad et al. |
| Japanese | + | Association with postmenopausal breast cancer | Suzuki et al. |
| Turkish | + | Association with premenopausal breast cancer | Ergul et al., Semenza et al. |
| Chinese, Thai, Pakistani, Syrian | - | Lack of association with the risk of breast cancer | |
| Meta-analysis | + | Shrubsole et al., Sangrajrang et al., Cheng et al., Akram et al., Lajin et al. | |
| East Asian | + | Association with the breast cancer risk | Qi et al., Zhong et al. |
| East Asian | + | Association with postmenopausal breast cancer | Zhong et al. |
| Asians | Association with the breast cancer risk | Rai, Kumar et al., Xie et al. | |
| Caucasians | - | Lack of association with the risk of breast cancer | Qi et al., Zhong et al. |
The Role of MTHFR C677T Polymorphism in Susceptibility to Breast Cancer in Various Populations
There are several studies from Asian populations reporting the influence of MTHFR C677T variants in susceptibility to breast cancer, but with inconsistency. Three studies from China found an association between MTHFR 677TT genotype with increased breast cancer risk (32-34). Among Japanese, the MTHFR 677TT genotype was associated with the risk of breast cancer in post-menopausal women (27). Also, case-control studies from Iran and Jordan suggested an association between MTHFR C677T polymorphism with the risk of breast cancer (35, 36). Two further studies from Turkey observed the increased risk of breast cancer in pre-menopausal women in the presence of MTHFR C677T variant (37, 38). Furthermore, more recent studies from Turkish population indicated that the MTHFR C677T was a risk factor for breast cancer (39). Also, in a population from Kazakhstan, MTHFR C677T increased the risk of breast cancer (40). It has been suggested that the MTHFR C677T could be an independent risk factor for sporadic breast cancer through the induction of oxidative stress among Indians (41). However, among south-eastern Asian populations, there exist studies from Chinese (42) and Thai women (43, 44) reporting the absence of association between MTHFR C677T polymorphism and the breast cancer risk. Moreover, in Pakistani women, no association was detected between MTHFR C677T and the risk of sporadic breast cancer (45). Besides, MTHFR C677T was not significantly associated with the risk of breast cancer in Syrian women (46). In 2 meta-analyses, strong association was detected between this polymorphism and the risk of breast cancer in East Asian population. However, the meta-analysis did not find such association in Caucasian population (47, 48). The latter meta-analysis suggested the risk of breast cancer was significantly associated with postmenopausal status (48). Also, 3 recent meta-analyses suggested a significant relation between MTHFR 677TT genotype and breast cancer risk in Asian populations (49-51). The association of MTHFR C677T polymorphism with hypomethylation of DNA might suggest a role for this polymorphism in development of cancer.
According to the literature, it seems the MTHFR C677T is associated with the risk of breast cancer among Asian populations, especially East Asians, but not in Caucasian populations. The inconsistent findings of association between the MTHFR C677T in various populations may underlie differences in ethnicity, lifestyle, and disease prevalence as well as possible limitations due to the relatively small sample size. There are a wide variation in the T allele frequencies of control resources in Asians (0.396), Indians (0.132), Caucasians (0.326), Middle Eastern countries (0.201), and Africans (0.196) that might account for the discrepancy in association between the MTHFR C677T polymorphism and cancer risk in different ethnic groups (51).
3.2. MTHFR A1298C Polymorphism
The MTHFR A1298C polymorphism (Glu429Ala, rs1801131) is located in exon 7. This point mutation in the MTHFR gene might affect the enzyme regulation since the activity of the enzyme decreases in the presence of this mutation (15).
Some reports from various ethnic groups have investigated the role of MTHFR A1298C polymorphism in susceptibility to breast cancer (Table 2). Among Canadians, Americans, south-eastern European, German, west Siberian region of Russia, and Brazilian women, the MTHFR A1298C was not associated with the risk of breast cancer (20, 21, 24, 28, 30, 31, 52, 53). However, among women from Kazakhstan, the MTHFR A1298C polymorphism reduced the risk of breast cancer (40). Also, in Americans, the MTHFR 1298C allele was inversely associated with the risk of breast cancer (9). The presence of MTHFR A1298C polymorphism might result in lower enzyme activity with increased availability of 5, 10-methylenetetrahydrofolate and reducing the uracil misincorporation (54).
| Population | Association (+/-) | Interpretation | Reference |
|---|---|---|---|
| Canadians, Americans, South-eastern European , Russian, German, Brazilian | - | Lack of association with the risk of breast cancer | Xu et al., Platek et al., Papandreou et al., Kotsopoulos et al., Henriquez-Hernandez et al., Justenhoven et al., Vainer et al., DeRoo et al., Ma et al. |
| Americans, Kazakh | + | Associated with reduced breast cancer risk | Chen et al., Akilzhanova et al. |
| Asians | |||
| Chinese, Thai, Jordanian | - | Lack of association with the risk of breast cancer | He et al., Awwad et al., Shrubsole et al., Sangrajrang et al., Cheng et al. |
| Iranian, Turkish, Pakistani, Syrian | + | Increased the risk of breast cancer | Hosseini et al., Ergul et al., Ozen et al., Akram et al., Lajin et al. |
| Meta-analyses | - | Lack of association with the risk of breast cancer | Zhong et al., Rai |
The MTHFR A1298C Polymorphism and the Risk of Breast Cancer in Various Populations
Among south-eastern Asians (China and Thailand) (34, 42-44), and in Asian population of Jordan, the MTHFR A1298C did not affect the risk of breast cancer (36). However, studies from some Asians countries, especially from Middle East countries including Iran, Turkey, Pakistan, and Syria reported an association between breast cancer risk and the MTHFR A1298C polymorphism that suggested the C allele of this polymorphism could be a risk factor for breast cancer (35, 37, 39, 45, 46). In 2 recent meta-analyses, the MTHFR A1298C polymorphism was not a risk factor for susceptibility to breast cancer (48, 49).
Reports from various populations indicate that the MTHFR A1298C polymorphism might not play a role in susceptibility to breast cancer.
3.3. MTHFR C677T and MTHFR A1298C Synergism and Breast Cancer
Among pre- and post-menopausal American women, no association was found between breast cancer risk for the combined genotype or for multiple alleles of MTHFR C677T and A1298C polymorphisms (21). However, in other study among Americans, the combination of MTHFR C677T and A1298C polymorphisms increased the risk of breast cancer in post-menopausal women (55). Also, in a meta-analysis conducted by Zhong et al. haplotype analysis demonstrated that MTHFR 677T/1298C compared to 677C/1298A haplotype significantly associated with the breast cancer risk in overall analysis and in Caucasians. They indicated that the haplotype 677C/1298C may be protective than wild haplotype 677C/1298A in East Asians (48). However, among Taiwanese women, the presence of combined genotypes of 677CT+TT and 1298AC+CC reduced the risk of cancer in women with lower level of plasma folate (56). They suggested that although circulating folate levels (5-methyl THF) tended to be low, the pool of intracellular 5, 10-methylene THF is increased since there is the reduced MTHFR activity in the presence of both polymorphisms of C677T and A1298C. Increased intracellular pools of 5, 10-methylene THF could increase the availability of thymine and, thereby, enhance DNA stability (56). Similarly, in a south-eastern European population, an association was detected for the MTHFR C677-MTHFR A1298 haplotypes with breast cancer risk (24).
Due to the presence of limited studies that have examined the epistatic interaction of both MTHFR polymorphisms in the risk of breast cancer, the role of MTHFR C677T-MTHFR A1298C haplotypes could not establish in the susceptibility to breast cancer. Although, it seems the concomitant presence of mutant alleles of MTHFR 677T and 1298C might be involved in susceptibility to breast cancer.
3.4. MTHFR Variants, Folate Intake, and B Vitamins in Breast Cancer
The MTHFR has a critical role in the one-carbon metabolizing genes pathway. It seems reduced formation of methyl donors increases the risk of breast cancer (55). It has been suggested that the risk of breast cancer can reduce in relation to intake of dietary folate and related B vitamins (B1, B2, B3, B6) and suboptimal folate metabolism increases the risk of breast cancer (9).
B vitamins (B1, thiamin; B2, riboflavin; B3, niacin; B6, pyridoxine; B9, folate; B12, cobalamin) play important roles in cell metabolism, and some of these vitamins are cofactors involved in the one carbon pathway. Among Taiwanese women, the plasma level of folate was inversely associated with the risk of breast cancer. Expanded intracellular pools of 5, 10-methylene THF could increase the availability of thymine and, thereby, enhance DNA stability (56). Also, in Caucasian, the B1 and B3 vitamins had a beneficial effect on survival of women with breast cancer (57).
Among American women, carrier of MTHFR 677TT genotype, who consumed the lowest levels of dietary folate the risk of breast cancer, was higher than those carriers of 677CC genotype with high folate intake (9). Moreover, in Chinese women, the MTHFR 677T allele was associated with increased risk of breast cancer in individuals with low folate intake, vitamin B6, and vitamin B12 (33). Likewise, another study from China confirmed the concomitant presence of MTHFR 677TT genotype and low folate intake increased the risk of breast cancer (42). Furthermore, among women from Saudi Arabia, no relationship was detected between the MTHFR C677T mutation and the risk of breast cancer, but in women with low folate intake and MTHFR 677TT genotype the risk of breast cancer increased (58). It seems individuals with the MTHFR 677TT genotype and low folate intake are susceptible to the risk of breast cancer due to the presence of higher levels of homocysteine, lower levels of methylated folates and, therefore, reductions in genomic DNA methylation (42). Folate deficiency increases DNA rupture, chromosome damage, and formation a micronucleus in lymphocytes (33), and MTHFR C677T and A1298C variants alter the levels of folate and homocysteine (33). However, in a prospective Swedish study, high plasma folate concentration was associated with increased risk of post-menopausal breast cancer in women carrier of the MTHFR 677T allele, indicating the role of MTHFR gene variants in complex relation between folate and cancer (59). It has been suggested that folate could prevent the development of tumors before pre-neoplastic lesions, but conversely increases tumorigenesis once lesions have been established (53). Among Chinese women (34), the absence of significant interaction between MTHFR C677T polymorphism and folate intake in the risk of breast cancer was reported. Also, a study from Brazil did not detect an association between MTHFR C677T polymorphism and dietary intake of folate, vitamin B6, and vitamin B12 with the risk of breast cancer (53) (Table 3).
| Population | Association (+/-) | Interpretation | Reference |
|---|---|---|---|
| American, Chinese, Saudi Arabian | + | Increased risk of breast cancer in carriers of MTHFR 677TT genotype with low folate intake | Chen et al., Jiang-hua et al., Shrubsole et al., Alshatwi et al. |
| Swedish | + | Increased risk of breast cancer in carriers of MTHFR 677T allele with high folate intake | Ericson et al. |
| Chinese, Brazilian | - | The absence of interaction between MTHFR C677T polymorphism and folate intake in the risk of breast cancer | He et al., Ma et al. |
The MTHFR Polymorphisms, Folate Intake and the Risk of Breast Cancer in Various Populations
In a study from Cyprus, neither MTHFR C677T nor MTHFR A1298C was associated with the risk of breast cancer. However, Mediterranean diet (a diet with frequent consumption of olive oil, the high intake of fruit, vegetables, legumes, cereals, bread and nuts, the moderate or low amounts of dairy products, fish, eggs and poultry, the low to moderate consumption of wine, and the low amounts of red meat) indicated that the high consumption of Mediterranean diet reduced the risk of breast cancer in individuals with the MTHFR 1298CC genotype (60) (Table 3).
Although many studies have suggested that the low dietary intake of folate along with MTHFR 677TT genotype increased the risk of breast cancer, some studies did not find such association. The absence of analysis of all genetic models and testing potential gene-gene and gene-diet interactions could affect the inconsistent findings. Moreover, more significance of impacts of environmental factors among genetically susceptible individuals might be considered for these variations. Differences in allelic frequency of MTHFR gene variants among various ethnic groups, the familial and early onset cancer, dietary factors, estrogen exposure, smoking status, and alcohol consumption are factors affecting the susceptibility to breast cancer.
4. Conclusions
Carcinogenesis, as a multi-factorial and multi-step process, involves various genetic alterations along with the effects of environmental factors. Due to the high prevalence of polymorphisms in the MTHFR gene among populations, the studies on relationship between these polymorphisms and the risk of breast cancer might help identify factors affecting the disease outcomes.
According to the available studies, it seems the variants of MTHFR C677T might be associated with the risk of breast cancer among Asians. The lower activity of the MTHFR enzyme in the presence of 677T allele with reduced availability of folate may result in uracil misincorporation in the DNA and cancer development. Changes in methylation of DNA and hypomethylation could modify the DNA conformation and gene expression. It seems the MTHFR A1298C polymorphism might not be a susceptibility factor for breast cancer.
The absence of establishment of a role for MTHFR haplotypes in susceptibility to breast cancer could be due to the presence of inconsistent findings in limited reports that studied the effect of synergism between both MTHFR C677T and A1298C variants in the risk of breast cancer. Although, many studies have suggested that low dietary intake of folate along with MTHFR 677TT genotype increased the risk of breast cancer but some studies did not find such association. Different ethnic background with differences in allelic frequency of MTHFR gene, small sample size, stage of cancer, the familial and early onset cancer, the influence of environmental factors including diet and life style, the absence of analysis of all genetic models and testing potential gene-gene and gene-diet interactions in association studies might affect the role of combined MTHFR gene polymorphisms and interaction between MTHFR polymorphisms with folate intake in susceptibility to breast cancer. To establish the role of MTHFR haplotypes in relation to folate intake in susceptibility to breast cancer, more studies with adequate sample size that investigate the role of MTHFR gene polymorphisms along with dietary intake of folate and B vitamins in the risk of breast cancer are necessary.