1. Background
The incidence of colorectal cancer, as one of the leading causes of cancer-related mortality worldwide, has been increasing over the last 25 years in Iran. Following cancers of breast and stomach, it is the third most common cancer among Iranian people with the annual incidence of more than 7100 cases (1).
Lymphovascular invasion (extension of tumor cells into lymphatic and/or blood vessels, LVI) and perineural invasion (neoplastic invasion of nerves and nerve sheath, PNI) are relatively common in various malignancies including colorectal cancers and have been shown to have prognostic significance.
2. Objectives
The aim of this study was to identify the clinical and pathological variables associated with LVI and PNI in colorectal cancer.
3. Methods
In this observational cross-sectional study, the records of patients with the diagnosis of colorectal, who had undergone an operation at Milad General Hospital (Tehran, Iran) between 2012 and 2017, were reviewed. All patients, whose pathology reports and treatment records were available at Milad Hospital, were included in the study (all available cases) and cases with incomplete records and missing necessary data were excluded from the study.
Relevant demographic, pathological, and surgical data, including age, gender, tumor location, maximum tumor size, pathologic Tumor, Node, Metastasis (TNM) stage, and grade and number of removed lymph nodes were extracted from medical records.
Tumor stages were defined according to the 7th edition of American Joint Committee on Cancer (AJCC) TNM staging system as follows: T1: tumor invades submucosa; T2: tumor invades muscularis propria; T3: tumor invades through the muscularis propria into the pericolorectal tissues; T4: tumor invades through the visceral peritoneum or directly invades or adheres to other adjacent organs or structures (2). Tumor grading was assessed according to the World Health Organization guidelines (3). This study was approved by the Ethics Committee of Milad Hospital.
3.1. Statistical Analysis
The chi-square test was used to evaluate the primary association among independent variables with LVI (0 = No, 1 = Yes) and PNI (0 = No, 1 = Yes). Adjusted binary logistic regression models were utilized to test the effect of the independent variables on both LVI and PNI in separate models. Afterward, a mixed variable of LVI and PNI was built by 3 categories of 0 = subjects, who had no LVI and PNI, 1 = subjects, who had at least one of them, and 2 = subject, who had both PNI and LVI. Multinomial logistic regression models were conducted to examine the association between independent variables and mixed variables. Due to the small sample size, T1 cases in binary logistic regression and T1, T2, and stage I cases in multinomial regression were excluded from the analysis. In the modeling process, the first group of dependent variables was considered as the reference except for the AJCC stage. Statistical analysis was performed, using SPSS version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, version 23.0. Armonk, NY: IBM Corp). P values of ≤ 0.05 were considered as statistically significant.
4. Results
In total, 547 patients (374 cases of colon cancer and 173 cases of rectal cancer) enrolled in the study. The results of the study are shown in Tables 1 to 3.
| Variables | LVI (N = 547) | PNI (N = 547) | ||||
|---|---|---|---|---|---|---|
| No | Yes | P Value | No | Yes | P Value | |
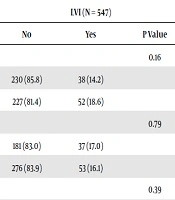
| Age, y | 0.16 | 0.08 | ||||
| < 60 | 230 (85.8) | 38 (14.2) | 195 (72.8) | 73 (27.2) | ||
| ≥ 60 | 227 (81.4) | 52 (18.6) | 184 (65.9) | 95 (34.1) | ||
| Gender | 0.79 | 0.71 | ||||
| Female | 181 (83.0) | 37 (17.0) | 153 (70.2) | 65 (29.8) | ||
| Male | 276 (83.9) | 53 (16.1) | 226 (68.7) | 103 (31.3) | ||
| Location of tumor | 0.39 | 0.15 | ||||
| Colon | 309 (82.6) | 65 (17.4) | 252 (67.4) | 122 (32.6) | ||
| Rectum | 148 (85.5) | 25 (14.5) | 127 (73.4) | 46 (26.6) | ||
| Maximum tumor size, cm | 0.009 | 0.14 | ||||
| < 5 | 223 (87.5) | 32 (12.5) | 183 (71.8) | 72 (28.2) | ||
| ≥ 5 | 205 (78.8) | 55 (21.2) | 171 (65.8) | 89 (34.2) | ||
| Depth of tumor invasion | 0.01 | < 0.001 | ||||
| T1 | 4 (100.0) | 0 | 4 (100.0) | 0 | ||
| T2 | 61 (96.8) | 2 (3.2) | 61 (96.8) | 2 (3.2) | ||
| T3 | 238 (81.2) | 55 (18.8) | 198 (67.6) | 95 (32.4) | ||
| T4 | 148 (82.7) | 31 (17.3) | 111 (62.0) | 68 (38.0) | ||
| AJCC stage | < 0.001 | < 0.001 | ||||
| I | 49 (98.0) | 1 (2.0) | 49 (98.0) | 1 (2.0) | ||
| II | 222 (95.7) | 10 (4.3) | 190 (81.9) | 42 (18.1) | ||
| III | 161 (74.2) | 56 (25.8) | 120 (55.3) | 97 (44.7) | ||
| IV | 12 (37.5) | 20 (62.5) | 9 (28.1) | 23 (71.9) | ||
| Histological grade | < 0.001 | < 0.001 | ||||
| I | 217 (93.5) | 15 (6.5) | 197 (84.9) | 35 (15.1) | ||
| II | 216 (81.2) | 50 (18.8) | 163 (61.3) | 103 (38.7) | ||
| III | 14 (35.9) | 25 (64.1) | 10 (25.6) | 29 (74.4) | ||
Abbreviations: AJCC, American Joint Committee on Cancer; LVI, lymphovascular invasion; PNI, perineural invasion.
aData presented as frequency (%).
| Variables | LVI | PNI | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y | ||||
| < 60 (ref) | ||||
| ≥ 60 | 1.77 (0.98 - 3.22) | 0.057 | 1.49 (0.94 - 2.35) | 0.08 |
| Gender | ||||
| Female (ref) | ||||
| Male | 0.62 (0.34 - 1.13) | 0.11 | 1.11 (0.70 - 1.76) | 0.64 |
| Location of tumor | ||||
| Colon (ref) | ||||
| Rectum | 1.19 (0.62 - 2.25) | 0.59 | 1.19 (0.72 - 1.96) | 0.49 |
| Maximum tumor size, cm | ||||
| < 5 (ref) | ||||
| ≥ 5 | 1.61 (0.88 - 2.94) | 0.11 | 0.96 (0.60 - 1.52) | 0.86 |
| Depth of tumor invasion | ||||
| T2 (ref) | ||||
| T3 | 0.36 (0.04 - 3.13) | 0.36 | 0.08 (0.01 - 0.66) | 0.02 |
| T4 | 1.42 (0.76 - 2.67) | 0.27 | 0.79 (0.49 - 1.27) | 0.34 |
| AJCC stage | ||||
| IV (ref) | ||||
| III | 0.39 (0.01 - 8.02) | 0.54 | 0.70 (0.03 - 13.0) | 0.81 |
| II | 3.64 (0.19 - 68.14) | 0.38 | 2.93 (0.16 - 52.25) | 0.46 |
| I | 13.81 (0.67 - 283.68) | 0.08 | 6.32 (0.32 - 124.55) | 0.22 |
| Histological grade | ||||
| I (ref) | ||||
| II | 2.05 (1.02 - 4.13) | 0.04 | 3.21 (1.93 - 5.34) | < 0.001 |
| III | 23.87 (8.49 - 67.07) | < 0.001 | 14.05 (5.64 - 34.95) | < 0.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; LVI, lymphovascular invasion; PNI, perineural invasion.
| Variables | Unadjusted, OR (95% CI) | Adjusted, OR (95% CI) | ||
|---|---|---|---|---|
| At Least One | Both | At Least One | Both | |
| Age, y | ||||
| < 60 (ref) | ||||
| ≥ 60 | 1.08 (0.70 - 1.65) | 1.68a (1.0 - 2.81) | 1.15 (0.70 - 1.90) | 2.27a (1.12 - 4.59) |
| Gender | ||||
| Female (ref) | ||||
| Male | 1.34 (0.86 - 2.09) | 0.88 (0.53 - 1.47) | 1.49 (0.89 - 2.51) | 0.59 (0.29 - 1.19) |
| Location of tumor | ||||
| Colon (ref) | ||||
| Rectum | 0.99 (0.63 - 1.55) | 1.61 (0.89 - 2.89) | 0.89 (0.52 - 1.53) | 1.42 (0.65 - 3.08) |
| Maximum tumor size, cm | ||||
| < 5 (ref) | ||||
| ≥ 5 | 1.24 (0.80 - 1.92) | 1.81a (1.07 - 3.06) | 1.08 (0.65 - 1.78) | 1.55 (0.76 - 3.17) |
| Depth of tumor invasion | ||||
| T3 (ref) | ||||
| T4 | 0.89 (0.57 - 1.40) | 0.87 (0.51 - 1.49) | 0.85 (0.51 - 1.42) | 1.10 (0.54 - 2.25) |
| AJCC stage | ||||
| IV (ref) | ||||
| III | 2.96b (1.86 - 4.71) | 10.88b (4.73 - 25.0) | 3.44b (2.06 - 5.73) | 73.38b (19.21 - 280.16) |
| II | 4.92b (1.63 - 14.84) | 68.69b (21.66 - 217.79) | 5.20b (1.59 - 16.99) | 16.18b (6.24 - 41.92) |
| Grade | ||||
| I (ref) | ||||
| II | 2.40b (1.50 - 3.81) | 6.54b (2.99 - 14.33) | 2.17b (1.28 - 3.67) | 5.87b (2.23 - 15.46) |
| III | 7.98b (2.84 - 22.41) | 74.64b (24.69 - 225.64) | 8.54b (2.70 - 26.94) | 119.66b (28.42 - 503.80) |
Abbreviation: AJCC, American Joint Committee on Cancer.
aP < 0.05
bP < 0.01.
The prevalence of LVI and PNI in our study was 16.4% and 30.7%, respectively. There was no difference between the incidence of LVI+ or PNI+ in different genders in either colon or rectal cancer. Although there was a trend toward more cases of LVI+ in the right side of the colon compared to the left side and rectum, there was no statistically significant difference between LVI+ cases in the colon (17.4%) and rectum (14.5%) (P = 0.35).
Patients with positive LVI were significantly more likely to have a higher T-stage (P = 0.01), tumor size of ≥ 5 cm (P = 0.01), higher grade, and higher overall AJCC stage (P < 0.01 and P < 0.01, respectively). In the same way, patients with positive PNI were more likely to have higher T-stage, higher grade, and higher overall stage (P < 0.01, P < 0.01, and P < 0.01, respectively).
Using binary logistic regression, it was found that patients with LVI+ were more likely to have grade II or III diseases (OR = 23.87, CI = 8.49 - 67.07; and OR = 2.05, CI = 1.02 - 4.13). Patients aged 60 years old and over were more likely to have LVI than the reference group (CI = 0.98 - 3.22). Furthermore, binary logistic regression confirmed that PNI+ remained statistically significant for histological grade II and III and T3 compared with their reference group.
Using the adjusted multinomial logistic regression analysis, we found that the odds of having at least one of the LVI or PNI in grade II and III were significantly higher than the reference group (OR = 2.17, CI 95% = 1.28 - 3.67; and OR = 8.54, CI 95% = 2.70 - 26.94) and it was also true when having both PNI and LVI (OR = 5.87, CI 95% = 2.23 - 15.46; and OR = 119.66, CI = 28.42 - 503.80).
The chance of having at least one or both LVI and PNI in stage III was higher than the reference group (OR = 3.44, CI = 2.06 - 5.73; and OR = 73.38, CI = 19.21 - 280.16) and the same was found for having at least one or both LVI and PNI in stage II (OR = 5.20, CI = 1.59 - 16.99; and OR = 16.18, CI = 6.24 - 41.92).
Unadjusted multinomial logistic regression showed that the chance of having both LVI and PNI compared with the reference group was significantly higher for patients of ≥ 60 years old, grade II and III, tumor size of ≥ 5 cm, and stage II and III.
5. Discussion
The results of the present study showed that patients with LVI+ were more likely to have higher tumor grade, higher T-stage, larger tumor size, and higher overall stage of colorectal carcinoma. These results are in line with the results of many previous studies (4-6). We also found that the presence of PNI, regardless of the status of LVI, is associated with higher tumor grade, higher T-stage, and higher overall stage.
While some studies have demonstrated that LVI+ is not associated with age (4, 6-8) or is associated with younger age (9), in the current study, patients aged 60 years old and over were more likely to have LVI or PNI, which is similar to the results of Lim SB et al.’s study (10).
Similar to many previous studies, this study showed that LVI or PNI are not associated with gender and age (4, 6-8), while in Al-Sukhni et al.’s study, male gender was associated with more incidence of PNI (5).
For years, the prognostic significance of LVI in colorectal cancer was a subject of controversy. While many studies had considered its presence as a negative prognostic factor, some studies had demonstrated that LVI was of no prognostic significance for colorectal cancers (10). Recent studies have resolved this controversy to a great extent, as several researchers have shown that the presence of LVI in colorectal cancer is a strong stage-independent prognostic factor. LVI is now thought to be involved in the development of lymphatic metastasis and it is considered by many international guidelines as an independent indicator of progressive disease, which can negatively affect patients’ survival (11, 12). Based on the College of American Pathologists Consensus Statement, LVI is considered as a Category I factor, which has been definitively proven to be of prognostic importance based on evidence from multiple statistically robust published trials (13).
PNI refers to the process of neoplastic invasion of nerves and nerve sheath. In many malignancies, including cancers of head and neck, pancreas, colon, and rectum, PNI is a marker of decreased survival and poor outcome (7). PNI is a distinct pathologic entity that can be present in the absence of LVI.
Several studies have demonstrated that the presence of PNI in colorectal tumoral cells is associated with higher rates of locoregional recurrence and decreased survival (14-16).
In the literature, the prevalence of LVI and PNI has been reported to be 21% to 25% and 9.9% to 14% for LVI and PNI, respectively (5). However, it is worth noting that studies that specifically reviewed the pathology samples for the presence of these features have reported higher rates of LVI and PNI positivity (33% and 22%, respectively) (5). By re-evaluating the pathology slides of 50 patients with colorectal cancer, Harris et al. (12) found significant inter-observer variability in the diagnosis of LVI on hematoxylin and eosin (H & E) slides, which were worse in cases of large vessel invasion and did not improve by immunohistochemistry. Another study by reviewing 381 pathology samples showed that the detection of vascular invasion was related to the quality of pathology assessments such as the number of assessed tissue blocks (17). These results highlight the need for high‐quality pathology reporting, more accurate criteria, and standardized quality control for the evaluation of LVI and PNI as the presence of these features can change the course of clinical treatment.
In the present study, 16.4% and 30.7% of cases were found to be positive for LVI and PNI, respectively. While the prevalence of LVI+ in our samples was similar to that of the previous studies, we had a higher rate of PNI+ compared to similar studies, for which we have no explanation.
In conclusion, in this study, we assessed the clinical and pathological variables associated with LVI and PNI in patients with colorectal carcinoma, who have been treated at a referral general hospital in Tehran, Iran. The results showed that colorectal carcinomas with positive LVI or PNI are more likely to have a higher grade, higher T-stage, and higher overall stage, and PNI is an independent factor for advanced disease.