1. Introduction
Lymph node infarction (LNI) is an extremely rare finding which is a result of coagulative necrosis of the lymph node tissue due to benign or malignant conditions (1, 2). The link between this phenomenon and malignant lymphomas has been previously described (3-7). Among lymphomas, diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma are the most frequent pathologies associated with LNI (5, 8, 9). In this case report, we describe our experience dealing with a case of DLBCL with extensive LNI and an initial open excisional biopsy devoid of any evidence regarding lymphoma.
2. Case Presentation
A 33-year-old man was referred for his left submandibular mass. He had noticed a gradual enlargement since roughly three months prior to that time. The mass had been painless until a week before we visited him. The pain was dull and had increased over time. He had been administered two intra-muscular injections of betamethasone (4 mg/mL) which had temporarily led to a decrease in size of the mass. No dysphagia, odynophagia, dysphonia, dyspnea, coughing, nasal obstruction, tooth-ache or epistaxis had been present. There was no recent animal exposure. Family, drug or past medical/surgical history were unremarkable. On physical examination, three masses were palpated as follows: 1, 6 × 3 cm in the left submandibular region; 2, 4 × 2 cm in the left level II of the neck; and 3, 2 × 2 cm in the submental region. All three were relatively fixed and non-tender, without any skin changes. Oral cavity examinations as well as rigid nasopharyngoscopy and laryngoscopy findings were normal.
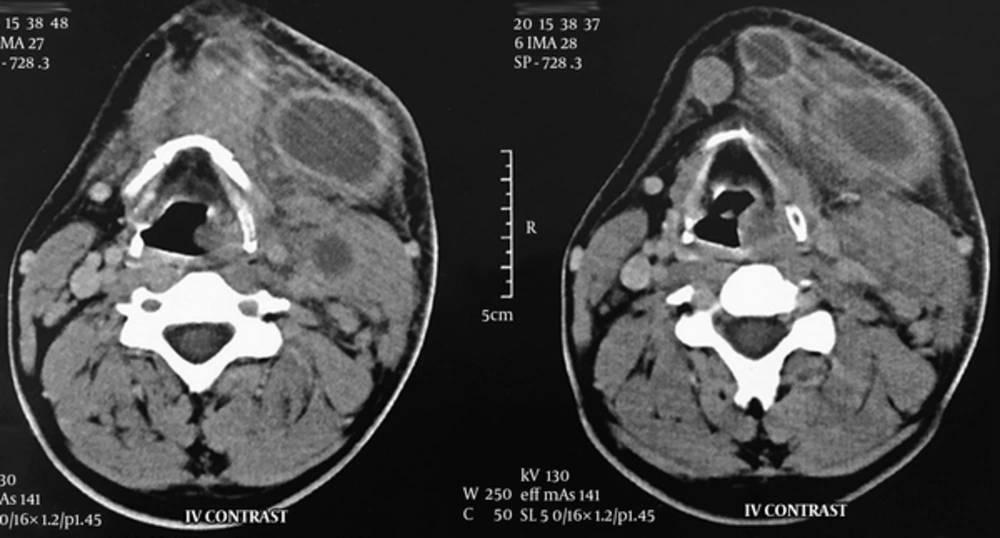
A neck computed tomography (CT) scan with intravenous (IV) contrast was performed. It revealed necrotic lymph nodes in the left submandibular, submental and level II regions with surrounding fat stranding without any primary source (Figure 1). Aspiration of the nodes showed no signs of infection; thus, we conducted a fine needle aspiration (FNA). Microscopic examination of the samples contained plasma cells, lymphocytes, neutrophils, tingible body macrophages, and nuclear dust, without any evidence of malignant cells. In complete blood count, the white blood cell count was 6,470 cells per microliter (mcL) with the following differential: 54.2% neutrophil, 30.6% lymphocyte, 9.7% monocyte, 4.9% eosinophil, and 0.6% basophil. Erythrocyte sedimentation rate (ESR) was 28 mm/hr and lactate dehydrogenase (LDH) amount was 432 IU/L. Anti-neutrophil cytoplasmic antibodies (ANCA) and rheumatoid factor (RF) values were both negative. Antinuclear antibody (ANA) titer was 1:40 dilution, which is the upper normal limit. The Mantoux tuberculin skin test was negative after 72 hours. Toxoplasma IgM antibody titer (3.0 IU/mL) was negative while IgG antibody titer (63.8 IU/mL) was positive. His chest radiography showed nothing abnormal.
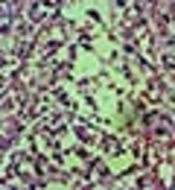
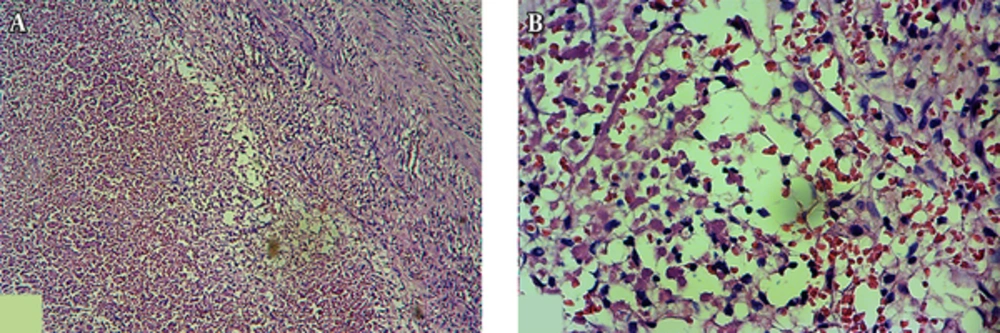
A trial of antibiotic therapy (IV clindamycin) was started. After 72 hours, size of the masses slightly decreased and the patient became pain free. Because of malignant features of the lymph nodes and chronic course of the disease, we performed an excisional biopsy of the submental lymph node. It was a yellowish, 2 × 2 cm lymph node with adhesion to the surrounding tissues. Surprisingly, microscopic examination of the lymph node revealed no viable cells (Figure 2). The periodic acid-Schiff (PAS) and Ziehl-Neelsen stainings were also negative.
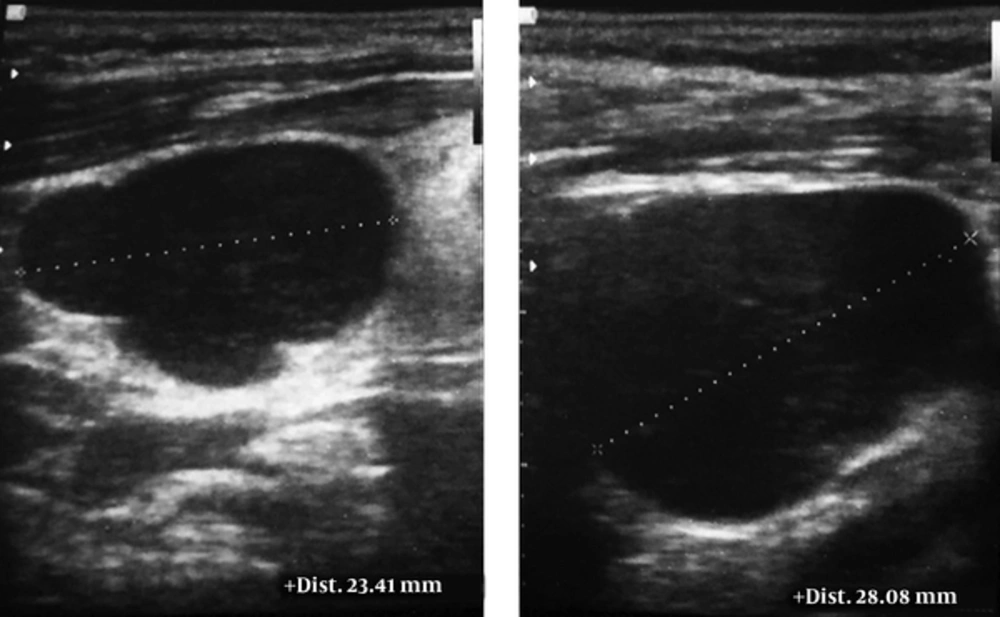
The patient was followed up for another week. During this period, two additional lymph nodes appeared at the right level II of his neck and supraclavicular regions. Ultrasonography detected multiple lymph nodes in the bilateral, anterior and posterior lymphatic chains. They were round, without hilum, and showing infiltrative features (Figure 3). This time, core needle biopsy was performed under ultrasonography guidance and small cylindrical specimens of pale tan tissues (1 × 0.5 × 0.2 cm) were obtained. Microscopic examination showed fibroconnective tissue, skeletal muscle, and fragments of lymphoid tissue, which was diffusely infiltrated by atypical hyperchromic cells containing prominent nucleoli and scant eosinophilic cytoplasm. Immunohistochemistry (IHC) study was positive for leukocyte common antigen (CD45), CD20, and Ki-67. Also, it was negative for epithelial membrane antigens and cytokeratin AE1/AE3. Thus, the primary diagnosis of B-cell lymphoma was established.
An excisional biopsy was performed on the submandibular lymph node which had a size of 2 × 2 × 1 cm and a pinkish tan color. Microscopic examination identified lymphomatous infiltration of fibrocollagenous stroma with diffuse growth pattern and areas of severe crush artifacts. In preserved areas, the infiltration was composed of large lymphoid cells with rounded to slightly irregular nuclei, having rather vesicular chromatin pattern and small nucleoli. In some areas the infiltration was associated with interstitial fibrosis. The IHC showed an intense diffuse positive reaction for CD20 and frequently for CD30. The BCL6 and CD10 were positive and ALK was negative. Furthermore, numerous CD3-positive T-lymphoid cells were seen in the background. The proliferative activity (Ki-67 expression) of the cells was 70%. Therefore, the definite diagnosis of germinal center type of DLBCL was achieved. Informed consent was obtained from the patient for the publication of the case details.
3. Discussion
In this case, four main diagnoses were considered: malignant lymphoma, metastatic squamous cell carcinoma, tuberculosis, and Kikuchi-Fujimoto disease (KFD). Nodal necrosis is frequently observed in metastatic squamous cell carcinoma of the head and neck. The presence of this finding should prompt to pursue searching for an undetected primary squamous neoplasm (10). In our case, we could not find any source for metastatic lymph node during diagnostic endoscopies and imaging. Another possibility, tuberculosis, was excluded by negative Mantoux test, chest radiography and acid fast staining. One of the differential diagnosis of necrotizing cervical lymph node is KFD, in which yellowish necrotic foci may rarely be distinguished on the cut surface of the node. Microscopic evaluations usually display paracortical foci with necrosis and histiocytic cellular infiltrate. The capsule may be infiltrated, and perinodal inflammation is common. The necrotizing process is often confined to circumscribed areas of eosinophilic fibrinoid material through irregular spreading of fragments of nuclear debris. Overt coagulative necrosis is not a diagnostic criterion of KFD (11). Here, we did not find any viable histiocytic cellular infiltrates which was not fully compatible with this disease. On the other hand, KFD is more prevalent among women and besides, extremely rare in Iran with only one reported case (12). Moreover, extensive necrosis and size of the lymph nodes were not typical for KFD (13).
Complete lymph node necrosis is an ominous sign for malignant lymphoma. In 1982, Cleary et al. reported that for 16 patients with complete necrotic lymph nodes, repeated biopsies after 2 days to 6 months revealed malignant lymphoma in 81% of cases (4). In our study, the microscopic assessment of FNA and the first excisional biopsy revealed no viable cells, whereas there was extensive necrosis. Thus, we performed core needle biopsy under ultrasonography guidance in order to choose the best lymph node before the second excisional biopsy. Novel studies have shown that core needle biopsy is a cost-effective procedure (14). Hu et al. reported that the core needle biopsy with guidance of ultrasonography have 85 to 87% accuracy for diagnosis of lymphoma (15). After the core needle biopsy and choosing the right lymph node, we performed a second excisional biopsy which yielded the diagnosis of DLBCL.
In this case report, we presented an unusual case of DLBCL with initial excisional biopsy showing diffused necrosis without evidence of malignant cells. As previously reported, it is highly recommended to closely follow up the patients with diffuse LNI, since a high proportion of them could be lymphoma (1). We also recommend using ultrasonography guided core needle biopsy before excisional biopsy to select the optimal lymph node and to avoid a non-diagnostic biopsy.