1. Background
Compared to primary brain tumors, brain metastases are a common and widespread problem with an upward trend in entire the world (1). The diagnosis of brain metastases is mainly based on imaging techniques; so, the diagnostic accuracy of the instrument and the radiologist’s experience have a central role in the accurate and timely diagnosis of the disease (2). Conventionally, magnetic resonance imaging (MRI) is used to assess the position and number of metastases. It is also used to determine the best surgical or radiological surgery plan, as well as to determine the response rate to the treatment (3, 4). Additionally, the main purpose of non-invasive imaging techniques in the assessment of brain tumor defects lies in the assessment of the tumor zone, tumor diameter, and its related tissue infiltration (5). Moreover, it is important to identify edema and dislocation that appeared in normal brain tissue due to the progression of the brain tumor. Overall, obtaining information on tumor staging and grading and the presence of necrosis can help to determine the best and effective treatment approach (6). Recently, modern MRI techniques have been extensively used to distinguish brain metastases from other brain tumors such as high-grade gliomas or abscesses (7). One of these techniques is the use of measuring the apparent diffusion coefficient (ADC) index of the lesion, which has recently been emphasized because of its diagnostic and even prognostic value (8, 9). In this regard, the assessment of the peritumoral area through diffusion-weighted imaging (DWI) was most desirable for differentiation between the metastatic tumors of the brain and primary tumors (10). Some metric parameters measured by DWI, such as the ADC index, directly evaluate the peritumoral region with high precision, and even the range of this specific index for brain metastases has also been discussed (11, 12). In fact, it seems that the interaction between the metastatic tissue of the brain and the tissue around the tumor provides the possibility of evaluating the response rate to anti-tumor regimens, as well as the likelihood of tumor invasion to other organs. Despite the importance of determining the ADC in tumor involved lesions, and especially in the peritumoral area, there is still no comprehensive study on its role in differentiating all types of brain metastatic brain lesions.
2. Objectives
The aim of this study was to determine the diagnostic performance of the ADC index in the discrimination of all types of metastatic brain lesions.
3. Methods
This study was conducted on 80 biopsy-proven patients with brain metastasis. Biopsy of brain lesions was performed either through brain surgery or stereotactic biopsy. All patients were referred to the neurosurgery department and underwent MRI and DWI imaging examination at Shohada-y-Tajrish Hospital affiliated by Shahid Beheshti University of Medical Sciences in Tehran, Iran between 2015 and 2019. We used the purposive sampling method for sampling the patients. The information of all cases with documented findings in MR imaging was entered in the MR imaging database. We excluded all subjects with the following criteria: the evidence of intratumoral hemorrhage, the history of chemotherapy or radiation therapy, the histological evidence of primary brain tumors, or no availability for digital data from MR imaging and DWI. The institutional review board at Shahid Beheshti University of Medical Sciences approved the study protocol (with the ethical code of IR.SBMU.MSP.REC.1397.277), and all patients gave written informed consent before imaging examination. All tumor characteristics, including the number and size of lesions, the origin of metastasis, the zone of metastasis, and the presence of necrosis or edema in the lesions were collected based on imaging techniques. MR imaging scans were done with a 1.5T superconducting system manufactured by Magnetom Vision; Siemens, Germany. The ADC measures were assessed at the two peritumoral and intratumoral regions with the following formula: ADC = -[ln (S/S0)]/b, where S indicated the signal intensities of the region of interest (ROI), S0 indicated the signal intensities of the ROI acquired through reference T2-weighted images, and b indicated the gradient b factor with a value of 1000 smm2. ADC maps were calculated on a pixel-by-pixel basis (13). In this regard, the two values of ADCmean and ADCmin were calculated. A number of metastases show hemorrhage and the T1-, T2- weighted, and b0 sequence were assessed for the evidence of intratumoral bleeding. Cases would also be excluded at this stage if > 50% of the tumor was affected by hemorrhage (14).
According to the results of the study by Hayashida et al. (13), with respect to the mean ADCmin of 1.81 ± 0.27 and 1.40 ± 0.36 for metastatic brain tumors with and without proper differentiation and considering the confidence of 0.95 and study power of 90%, a minimum number of samples acquired for performing the study was 15 for each two subgroups. To present the quantitative and numerical variables, the mean ± standard deviation and number (percentage) were used. The Kolmogorov-Smirnoff test was used to determine the normality of distribution. Categorical variables were compared, using the chi-square test and quantitative variables by analysis of variance or Kruskal-Wallis H tests. The values of ADCmin and ADCmean in the differentiation of different types of tumor lesions were assessed by analyzing the ROC curve and in this regard, the best cut-off value and sensitivity and specificity of each parameter were determined. The software SPSS version 16.0 for windows (SPSS Inc., Chicago, IL) was used to analyze the data. P values ≤ 0.05 were considered statistically significant.
4. Results
Of 80 patients, 62.5% were female and 37.5% were male. Right-sided lesions were found in 60% and left-sided lesions in 40%. The single lesion was revealed in 38.8% and multiple lesions in 61.2%. The origin of metastasis was 40% and 22.5% in the breast and lungs, respectively. Metastasis was frequently related to the supratentorial region (78.8%) followed by in infratentorial region (17.5%). The size of legion more than 40 mm was found in 35%. Lesion necrosis was found in 80.0% and most of the patients (98.7%) had edema in their metastatic lesions (Table 1). The mean intratumoral ADCmin value was 694.24 ± 185.86 with the range of 316 to 1210 and the mean intratumoral ADCmean was 784.22 ± 189.04 with the range of 361 to 1307. The mean ADCmin and ADCmean in peritumoral lesions were also 1497.62 ± 238.26 (ranged from 483 to 1939) and 1592.28 ± 246.36 (ranged 578 to 2225), respectively. The mean of ADCmin and ADCmean values in both intratumoral and peritumoral zones, according to the source of metastasis, are presented in Table 2. There was no difference in the mean ADCmin and ADCmean values in both zones of lesions across the different sources of metastasis. Also, we showed no difference in ADCmin and ADCmean values between supratentorial and infratentorial lesions (Table 2). There was also no significant association between the ADCmin and ADCmean measures and the size of a malignant lesion. As shown in Table 2, no difference was found in ADCmin and ADCmean between the subgroups of lesions with and without necrosis; however, regarding the association between the values of two parameters and the presence of edema, more severe edema in peritumoral zone was related to significantly higher ADCmin and ADCmean values (Table 2). As summarized in Table 3, peritumoral ADCmin had the highest value for predicting breast lesions, as well as lung lesions as the source of metastasis. However, none of the ADC values could discriminate the source of brain metastasis.
| Variables | Values |
|---|---|
| Location of lesion | |
| Right | 48 (60.0) |
| Left | 32 (40.0) |
| Number of lesions | |
| 1 | 32 (40.0) |
| 2 | 16 (20.0) |
| 3 | 8 (10.0) |
| 4 | 7 (8.8) |
| More | 17 (21.3) |
| Source of metastasis | |
| Breast | 32 (40.0) |
| Lung | 18 (22.5) |
| Others | 30 (37.5) |
| Place of metastasis | |
| Infratentorial | 14 (17.5) |
| Supratentorial | 63 (78.8) |
| Other zones | 3 (3.8) |
| Size of lesion, mm | |
| < 20 | 9 (11.3) |
| 20 to 40 | 43 (53.8) |
| > 40 | 28 (35.0) |
| Necrosis in lesion | |
| None | 16 (20.0) |
| Mild | 30 (37.5) |
| Severe | 34 (42.5) |
| Edema in lesion | |
| None | 1 (1.3) |
| Mild | 24 (30.0) |
| Severe | 32 (40.0) |
Characteristics of Metastatic Brain Lesions
| Characteristics | Intratumoral ADC | Peritumoral ADC | ||
|---|---|---|---|---|
| ADCmin | ADCmean | ADCmin | ADCmean | |
| Source of metastasis | ||||
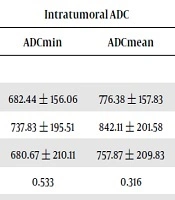
| Breast | 682.44 ± 156.06 | 776.38 ± 157.83 | 1495.1 ± 162.44 | 1591.40 ± 165.09 |
| Lung | 737.83 ± 195.51 | 842.11 ± 201.58 | 1551.0 ± 182.34 | 1633.28 ± 193.34 |
| Others | 680.67 ± 210.11 | 757.87 ± 209.83 | 1530.1 ± 217.58 | 1632.55 ± 227.83 |
| P value | 0.533 | 0.316 | 0.585 | 0.669 |
| Place of metastasis | ||||
| Infratentorial | 733.36 ± 207.46 | 826.79 ± 201.28 | 1434.93 ± 145.88 | 1533.00 ± 158.23 |
| Supratentorial | 691.52 ± 183.88 | 780.17 ± 189.68 | 1540.68 ± 196.62 | 1637.38 ± 203.80 |
| Other zones | 568.67 ± 44.50 | 670.67 ± 17.04 | 1538.00 ± 67.80 | 1593.33 ± 68.06 |
| P value | 0.372 | 0.407 | 0.166 | 0.196 |
| Size of lesion, mm | ||||
| < 20 | 690.68 ± 122.12 | 757.44 ± 123.27 | 1402.56 ± 242.84 | 1490.33 ± 271.38 |
| 20 to 40 | 695.02 ± 222.18 | 776.26 ± 229.89 | 1528.80 ± 182.30 | 1634.65 ± 192.27 |
| > 40 | 712.68 ± 135.18 | 805.07 ± 130.76 | 1548.89 ± 169.57 | 1631.64 ± 162.94 |
| P value | 0.541 | 0.747 | 0.119 | 0.119 |
| Necrosis in lesion | ||||
| None | 685.56 ± 231.94 | 779.06 ± 229.12 | 1538.06 ± 224.21 | 1634.47 ± 232.49 |
| Mild | 669.80 ± 187.86 | 754.43 ± 194.02 | 1523.32 ± 182.26 | 1639.71 ± 198.63 |
| Severe | 719.88 ± 161.27 | 812.94 ± 164.12 | 1512.12 ± 181.45 | 1589.88 ± 177.92 |
| P value | 0.555 | 0.468 | 0.902 | 0.569 |
| Edema in lesion | ||||
| None | 677.26 ± 157.18 | 781.04 ± 159.96 | 1410.21 ± 212.99 | 1549.68 ± 260.45 |
| Mild | 706.29 ± 210.40 | 804.21 ± 210.71 | 1498.96 ± 148.25 | 1587.88 ± 151.54 |
| Severe | 702.38 ± 190.90 | 777.78 ± 195.72 | 1617.55 ± 148.99 | 1686.55 ± 151.43 |
| P value | 0.845 | 0.865 | 0.001 | 0.027 |
The Mean ADC Values According to the Characteristics of Lesions
| Index | Breast vs. Others | Lung vs. Others | Others vs. Breast and Lung |
|---|---|---|---|
| Intratumoral ADCmin | |||
| AUC | 0.507 | 0.559 | 0.552 |
| Cut-off point | 656.5 | 681.5 | 713.5 |
| Sensitivity | 0.63 | 0.61 | 0.63 |
| Specificity | 0.46 | 0.47 | 0.59 |
| Intratumoral ADCmean | |||
| AUC | 0.514 | 0.595 | 0.585 |
| Cut-off point | 772.5 | 778.5 | 0.801 |
| Sensitivity | 0.63 | 0.67 | 0.63 |
| Specificity | 0.50 | 0.52 | 0.54 |
| Peritumoral ADCmin | |||
| AUC | 0.602 | 0.597 | 0.530 |
| Cut-off point | 1565.0 | 1510.5 | 1466.0 |
| Sensitivity | 0.70 | 0.72 | 0.62 |
| Specificity | 0.51 | 0.51 | 0.31 |
| Peritumoral ADCmean | |||
| AUC | 0.583 | 0.577 | 0.526 |
| Cut-off point | 1654.5 | 1611.5 | 1578 |
| Sensitivity | 0.70 | 0.67 | 0.62 |
| Specificity | 0.51 | 0.51 | 0.35 |
The Value of ADCs to Predict the Type of Metastatic Brain Lesions
5. Discussion
In brain metastatic lesions, peritumoral edema assessed by advanced imaging modalities is reported to have infiltration by neoplastic cells; however, determining the tumor border is still inaccurately depicted even by applying such modalities. More importantly, the ADC values of this area for predicting tumor-related characteristics such as diametric and morphologic characteristics of lesions, as well as their primary source, remain uncertain. In the current study, we aimed at assessing the value of ADC values in both intratumoral and peritumoral areas to determine the metastatic brain tumor source and to predict the morphological characteristics of the tumor. As shown by the results, although the ADC values of the peritumoral area were strongly associated with the grade of edema in this area, none of the values could accurately predict the origin of metastasis. In other words, determining the ADCmin and ADCmean in the intratumoral area could not be indicators for lesion characteristics such as size and location of the lesion, the presence of necrosis or edema, as well as the source of the metastatic tumor such as breast or lung. Similarly, the ADCmin and ADCmean in the peritumoral area could be an effective predictor for determining the source of metastasis and also diameter, location, or necrotic nature of the tumor and only can predict the edematous pattern of the lesion. Although it was not our aim, the ADC values have been shown to be strong discriminative between metastases and primary tumors (15). However, all studies have been unanimously agreed that ADCs and DWIs are not helpful to determine the existence of peritumoral neoplastic cell infiltration (16). In a study by Caravan et al. in 2018 (9), ADCmin and ADCmean were measured in both intratumoral and peritumoral areas and showed that the ADCmin in intratumoral regions in primary brain tumors, such as high-grade glioma, was far higher than metastatic tumors. Also, ADCmin in the peritumoral region in the primary brain tumors was far less than that of metastatic tumors; however, they did not assess the value of this parameter to predict the source of metastatic tumors. Contrary to the results of this, in Zakaria et al.’s study (14), ADC values related to brain metastases sourced by primary brain tumors showed significantly higher ADCmin in tumors originated by the lung, non-small lung cancer, ovarian, and colorectal cancers compared to small cell lung cancer and melanoma. Also, although we could not find any association between ADC values in both intratumoral and peritumoral areas and morphological characteristics of tumors, in a study by Hayashida et al. (13), ADC values reflected tumor cellularity and its histological type. As a comprehensive interpretation of our findings and previous studies, the measurement of ADC values can effectively predict primary tumors from metastases and differentiate the grade of the tumor. However, determining the ADC values, particularly in peritumoral areas, may not predict the tumor morphology or its origin. In this regard, it seems that the application of other conventional modalities or imaging indices such as whole body scanning and magnetic resonance spectroscopy may be more useful.
In the present study, we attempted to assess the value of ADCs in both intratumoral and peritumoral areas to determine the metastatic brain tumor source and to predict the morphological characteristics of the tumor.
5.1. Conclusions
As the final conclusion, the ADC values in both intratumoral and peritumoral areas of the brain tumor metastatic lesion may not predict the assessment of tumor morphology or its origin. The values of ADCmin and ADCmean in the peritumoral area are an indicator of the severity of edema in this area.
.jpg)