1. Background
Breast cancer is the most prevalent type of cancer among women worldwide, which is identified as the most well-known reason for cancer mortality (1). Many of the current treatments for breast cancer, including surgery, radiation, and chemotherapy, have not been effective in diminishing mortality rates, but immunotherapy has become one of the greatest approaches to cancer treatment (2). Recently, researchers have offered a novel method for treating cancer called “immunotoxin therapy”, which is hopefully going to advance cancer therapy.
Immunotoxins are composed of a toxin that joins to antibodies targeting antigens existing on cancer cells’ surface (3). According to molecular analyses, breast cancer is classified into multiple intrinsic groups such as luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-positive, basal-like (triple-negative), erb-B2, and normal breast-like (4). Among these subtypes, nearly 15% to 20% of patients with breast cancer are HER2-positive as diagnosed by the overexpression of HER2 receptors and/or HER2 gene amplification.
HER2 is a 181 kDa transmembrane glycoprotein that belongs to the epidermal growth factor receptor (EGFR) family with tyrosine kinases activity. HER2 gene is located on chromosome 17q21 and encodes the HER2 receptor, which has proto-oncogene activity in normal breast cells (5). HER family (i.e., HER1, HER2, HER3, and HER4) plays a crucial role in cell proliferation, differentiation, and survival; however, unlike other members of the HER family, HER2 does not bind to any ligand or act as a co-factor for several ligands (6). However, HER2 can dimerize in two ways: (1) heterodimerization with other HER family members and (2) homodimerization when HER2 exists in high concentrations, especially in carcinoma.
Following the dimerization of HER2, phosphorylation of tyrosine residues occurs leading to the initiation of various intracellular signaling cascades such as PI3K/AKT and ERK/MAPK (aka: Ras/Raf/MEK/ERK) pathways that are associated with cell proliferation, apoptosis, migration, invasion, differentiation, and angiogenesis (7). HER2 overexpression results in enhanced malignancy recurrence, high risk of metastasis, common chemotherapy resistance, and endocrine therapies, which subsequently lead to poor prognosis and shortened survival. For this reason, HER2 is targeted in breast cancer treatment, and it is recognized that HER2-targeted therapies remarkably improve the survival of patients with HER2-positive breast cancer (8).
Trastuzumab (Herceptin) is one of the humanized monoclonal anti-HER2 antibodies used for targeting HER2-positive breast cancer (9). Although trastuzumab shows better improvement rates during therapy than other treatments do, patients have demonstrated resistance to trastuzumab, even when combined with other chemotherapy drugs (10). Based on the previous results, it is suggested that treatment with anti-HER2 antibody-targeted toxin is possibly more effective than the anti-proliferative antibody for patients (11).
Single-chain variable fragment (scFv) is a small antibody fragment composed of heavy (VH) and light (VL) chains, which are connected to one another through short flexible peptide linkers (about 10 - 25 amino acids) (12). ScFv has been used in different immunotoxins for its low molecular weight (∼1 kDa), high elasticity in chemical conjugation, low antigenicity, easy production, great tissue/cell penetration, and high biocompatibility, binding, and specificity. ScFv has been extended to transfer toxins, specifically to cancer cells (13).
Cytolethal distending toxins (CDTs) are identified as the first bacterial genotoxins that can cause DNA damage, DNA mutation, or trigger cancer, and they are generated by particular Gram-negative bacteria exhibiting DNase activity (14). Since CDTs belong to the AB toxin family, they comprise 3 subunits, including CdtA, CdtB, and CdtC. The complex of CdtA and CdtC B subunits attaches to cell surface receptors and permits the entrance of CdtB to the nucleus of the host cell and provokes DNA injuries. CdtB A subunit is an active segment causing double-strand breaks in host cells (15). For higher effectiveness, CdtB should be translocated to the nucleus. In addition, the CdtB is believed to have phosphatase activity that provokes apoptosis in T-cells. These functions of CdtB (phosphatase and/or DNase activities) in host cells lead to cell cycle arrest in the G2/M phase, distension, and ultimately, apoptosis (16).
2. Objectives
In the present study, we used in silico strategies to design chimeric constructs comprised of HER2-specific scFv, as a targeting molecule, and bacterial toxin segment obtained from Campylobacter jejuni (Cj-CdtB) connected to HER2-specific scFv via a flexible linker. A three-dimensional (3D) model for this chimeric protein was constructed and its structure, stability, solubility, and binding to HER2 were predicted and, then, evaluated, using in silico procedures.
3. Methods
3.1. Sequence Analysis and Construct Design
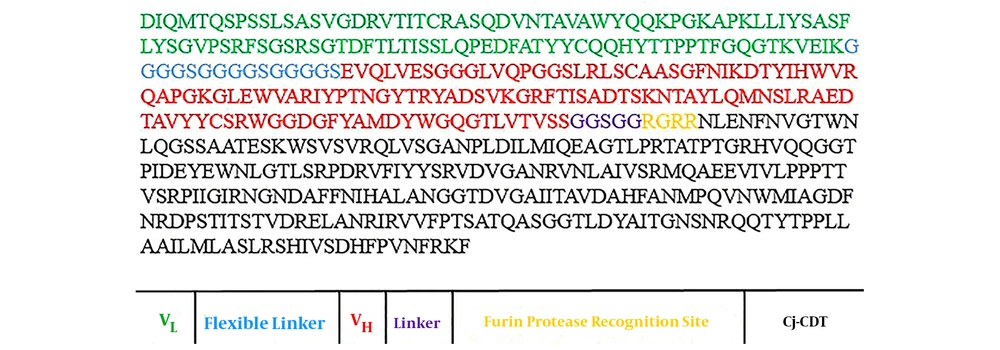
The amino acid sequences of Cj-CdtB and trastuzumab scFv, including VL and VH sequence alignments, were obtained from Uniprot (Uniprot ID: Q46101) and Protein Data Bank (PDB ID: 1n8z), respectively. First, VL and VH of Herceptin were bound by the GGGGSGGGGSGGGGS linker and constituted scFv. Afterward, the GGSGG sequence was selected as the optimal linker between the Cj-CdtB and scFv. Many hydrophobic linkers were considered to choose a suitable linker to preserve functionality and improve the normal structure of 3 sections of the recombinant protein (data not shown). In this construct, we also designed a Furin protease recognition site with RGRR amino acid sequence between the GGSGG linker and the Cj-CDT segment (Figure 1).
3.2. Secondary Structure Prediction
In this step, the secondary structure of the VL and VH alone and after scFv forming was analyzed, using the GORV server. Then, the secondary structure of scFv was compared before and after linking to Cj-CdtB via a short linker. In the end, the percentage of secondary structures in the final structure was calculated.
3.3. Physicochemical Properties and Solubility Prediction
Various physicochemical properties of the immunotoxin, including its molecular weight, theoretical isoelectric point (pI), net charge, instability index, aliphatic index, and grand average of hydropathicity (GRAVY) were analyzed, using ProtParam web server. The PROSO II online software was used for immunotoxin solubility prediction. PROSO II is a machine learning-based software that implements classification methods and mines in experimental data to evaluate the accuracy of coverage and solubility predictions.
3.4. Tertiary Structure Prediction, Refinement, and Validation
The 3D models for the structure of the chimeric structure were constructed, using I-TASSER. This combined platform was employed for protein structure analysis and function prediction. The best-predicted protein models were submitted to the GalaxyRefine server for protein structure refinement.
RAMPAGE and PROCHECK servers were used for the validation of 3D models. RAMPAGE and PROCHECK servers that evaluate the stereochemical properties of the protein structure showed the number of residues in favor and most favor regions and demonstrated the 3D model’s quality before and after refinement.
3.5. Allergenicity Prediction
AllergenFP is a bioinformatics tool based on the descriptor fingerprint used for the potential allergenicity prediction of chimeric structure.
3.6. mRNA Stability Prediction
RNAfold web server was employed to predict the mRNA secondary structure of the chimeric protein according to energy minimization.
3.7. Immunotoxin and HER2 Docking
The chimeric protein and HER2 docking were performed, using the ZDOCK server. This server can estimate protein-ligand docking. HER2 and the refined 3D structure of scFv + Cj-CdtB fusion protein, as a ligand in PDB format, were submitted to the ZDOCK server. Default parameters were applied to perform the estimations.
The method diagram is shown in Figure 2.
4. Results
4.1. Secondary Structure Prediction
The results of the GORV web server revealed that the connection of VL and VH via the flexible linker and constituting the scFv did not change their secondary structure (data not shown). Furthermore, the scFv secondary structure was not changed by the connection of Cj-CdtB and scFv via the short linkers and forming the scFv + Cj-CdtB (data not shown). The chimeric protein secondary structure contained 16.00% α-helix, 27.80% extended strand, and 56.20% random coil.
4.2. Physicochemical Properties and Solubility
The physicochemical features and the solubility of chimeric protein sequences were observed, using ProtParam and PROSO II, respectively. According to the ProtParam results, molecular weight, theoretical pI, and net charge were 53743.10, 8.90, and +5, respectively. Aliphatic index and GRAVY of the fusion protein were 74.90 and -0.250, respectively. scFv + Cj-CdtB was characterized as a stable protein (instability index = 38.15, the index below 40 means that the protein is stable), and based on the result of solubility prediction (> 0.5), our chimeric protein was demonstrated to be a soluble protein (solubility = 0.727).
4.3. A 3D Model Building, Refinement, and Validation
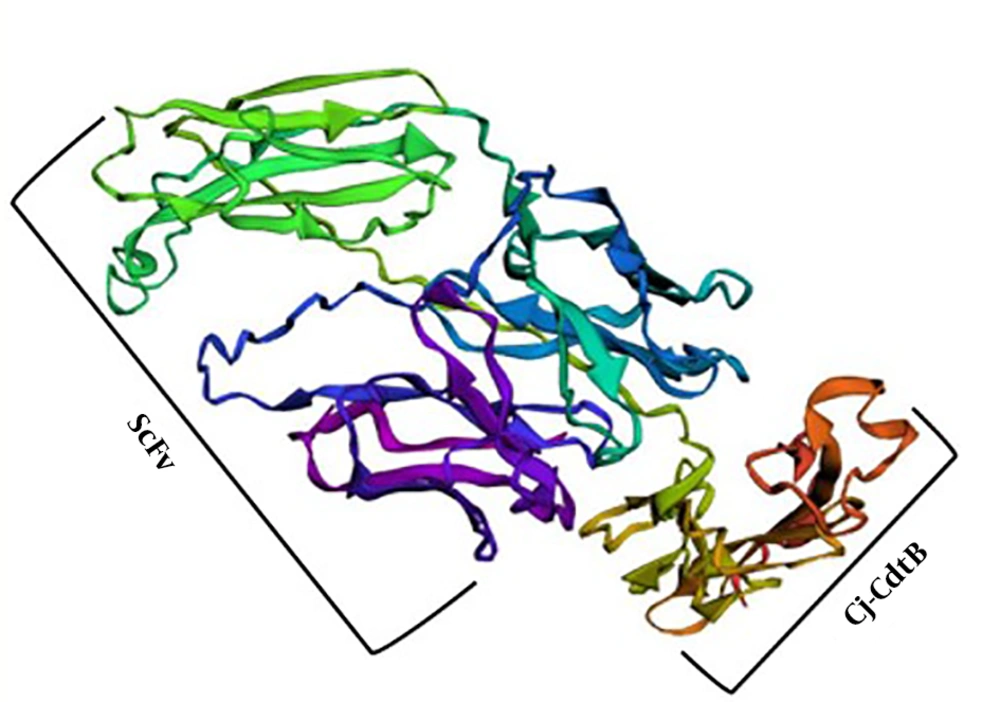
Five 3D models of the chimeric protein were constructed by the I-TASSER server, and the model with the highest confidence-score (c-score) was selected (Figure 3). C-scores were between -5 and 2. The higher value of the c-score shows more confidence and vice versa. This generated model was improved, using the Galaxy Refine server. Among the 5 refined models that were proposed by Galaxy Refine, the refined model with the most favored region in the Ramachandran plot was adopted. Then, the quality of unrefined and refined models was evaluated by RAMPAGE and PROCHECK and the comparison of Ramachandran plot before and after model refinement in terms of residence of residues was shown in Table 1.
| Before Refinement | After Refinement | |
|---|---|---|
| RAMPAGE | ||
| Number of residues in favored region | 346 (69.5) | 433 (86.9) |
| Number of residues in allowed region | 98 (19.7) | 49 (9.8) |
| Number of residues in outlier region | 54 (10.8) | 16 (3.2) |
| PROCHECK | ||
| Residues in most favored regions | 245 (58.9) | 315 (75.7) |
| Residues in additional allowed regions | 127 (30.5) | 82 (19.7) |
| Residues in generously allowed regions | 28 (6.7) | 14 (3.4) |
| Residues in disallowed regions | 16 (3.8) | 5 (1.2) |
aValues are expresses as No. (%).
4.4. Allergenicity Evaluation
AlgPred web server evaluation showed that the scFv + Cj-CdtB chimeric construct is not an allergen for the human body.
4.5. mRNA Stability
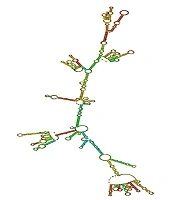
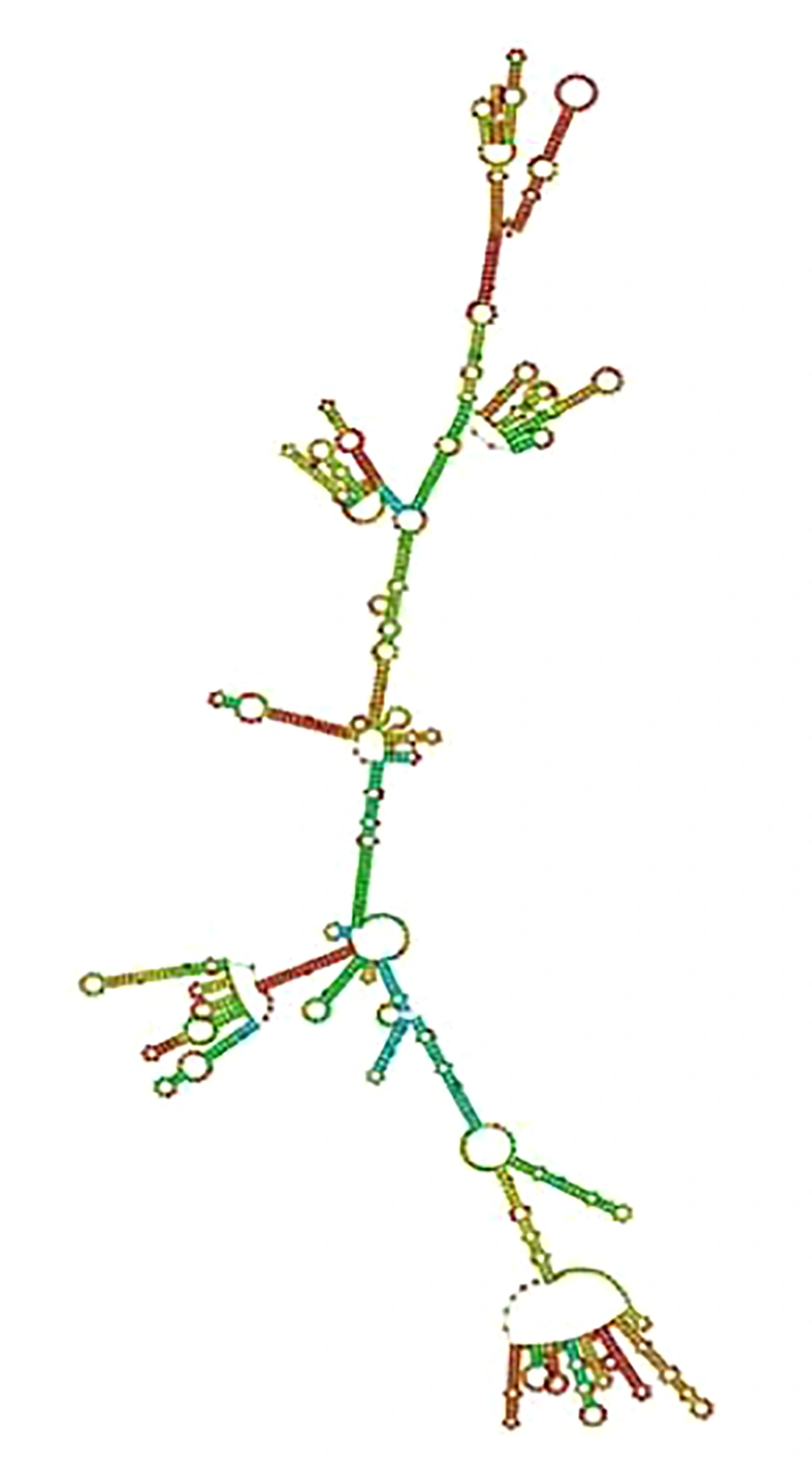
Based on the outcome of the RNAfold web server that calculates the free energy minimization in order to predict RNA secondary structures, the free energy of the thermodynamic ensemble was -667.58 kcal/mol (stable). When the mRNA is stable, its free energy value is negative. The secondary structure of the mRNA construct is shown in Figure 4.
4.6. Ligand Docking
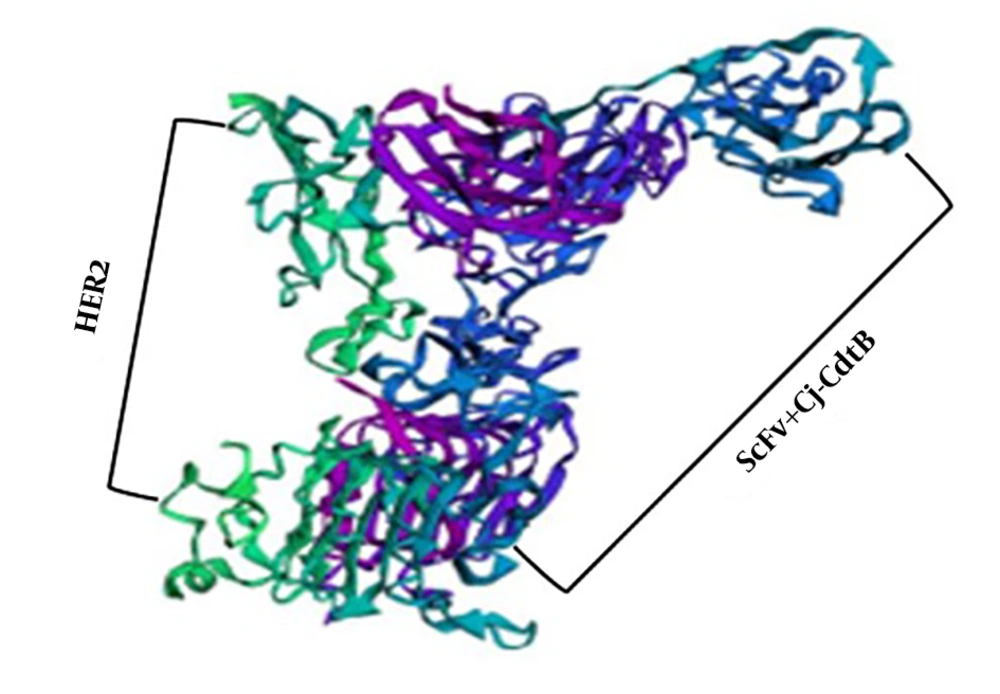
Ten 3D models of HER2 receptor and scFv + Cj-CdtB chimeric protein were generated by the ZDOCK server. The immunotoxin protein was bound to the HER2 receptor with high affinity and specificity. One of the best-visualized models for this docking is illustrated in Figure 5.
5. Discussion
Breast cancer is known as the most common malignancy among female adults of various races and ages, and it is the second cause of cancer mortality (1). According to numerous studies and observations, it has been stated that conventional therapies for breast cancer such as surgery, radiation, and chemotherapy have not decreased mortality rates (2). Among subtypes of breast cancer, it is revealed that HER2-positive patients demonstrate worse prognosis and shorter survival. Therefore, HER2, an overexpressed surface receptor on breast cancer cells, is selected for targeted therapy, and over the years, the survival of HER2-positive patients has increased (17). The first humanized monoclonal anti-HER2 antibody is trastuzumab (Herceptin). According to inquiries, it is declared that only 20% of patients with the overexpression of HER2 respond to trastuzumab. Although this drug improves HER2-positive patients’ condition and reduces metastasis, resistance to trastuzumab has been documented (18). Nowadays, immunotoxin therapy has attracted great consideration for its specificity and effectiveness on cancer cells. Immunotoxins obtain their toxicity from a protein toxin and their specificity from an antibody (3).
Nowadays, bioinformatics is changing biology and medicine (19). In the current study, we employed in silico procedures to design a special bio-conjugate (immunotoxin), including HER2-specific scFv derived from trastuzumab and a functional segment of Campylobacter jejuni cytolethal distending toxin (Cj-CdtB) for healing patients with breast cancer with increased HER2 receptors. Our immunotoxin design was similar to Sokolova et al.’s study in 2017. They utilize scFv fragment of the HER2-specific monoclonal antibody “trastuzumab” and PE40, which is a part of Pseudomonas exotoxin A (PE) for constructing a toxic module. This chimeric protein was an efficient immunotoxin against HER2 positive breast cancer cells in vitro and in vivo. Their results confirmed the powerful anticancer potential of scFv-PE40 (20).
At first, we created a scFv, using VL and VH of trastuzumab and attached these small segments of the antibody through a short peptide linker. In fact, the reason for scFv usage was its superior properties, including lower molecular weight, lower antigenicity, higher binding, and greater specificity compared to antibodies (13). In fact, VL and VH were bound together by the GGGGSGGGGSGGGGS linker and constituted scFv. The length and amino acid composition of the peptide linker is important in maintaining the scFv structure and stability. Typically, due to its flexibility, the linker peptide is about 3.5 nm (35°A) in length and contains hydrophobic amino acids such as glycine and serine residues (21). In order to create an efficient anticancer immunotoxin, the scFv was conjugated to Cj-CdtB, which is a subunit of the bacterial toxin obtained from Campylobacter jejuni (22). In order to create a linkage between scFv and Cj-CdtB, hydrophobic GGSGG amino acid was selected as the optimal flexible linker. Zhang et al. constructed two immunotoxins based on trastuzumab scFv and a cytotoxic drug DM1 called T-SA1-HAS-DM1 and T-SA2-HAS-DM1. T-SA1-HAS-DM1 showed potential anti-tumor activity and was distributed in xenograft models. Our immunotoxin design was similar to T-SA1-HSA. T-SA1-HAS consisted of VL and VH of trastuzumab, which was bound together by the GGGGSGGGGSGGGGS linker. Furthermore, the linker between scFv and HSA was GGSGG (23). Recently, Goleij et al. designed an immunotoxin that contained a scFv, whose VL and VH were bound together by the GGGGSGGGGSGGGGS linker. Their results showed that this scFv was stable and could bind to HER2 effectively (24). In accordance with this study, our scFv was also stable and docking of our immunotoxin and HER2 showed intense binding.
The efficiency of an immunotoxin is dependent on successful endocytosis, which is based on scFv and receptor interaction and internalization of the receptor-bound immunotoxin (25). The immunotoxin-HER2 complex is internalized via receptor-mediated endocytosis. After internalization, the complex can be sorted in early endosomes or in multivesicular bodies. Finally, HER2 is recycled to the membrane and the immunotoxin can go to Golgi (26). The cleavage of cytolethal distending toxin (CDT) is initiated by the furin protease of the Golgi system; then, it retrogrades to the endoplasmic reticulum (ER) and, ultimately, it is transported into the nucleus and leads to DNA damage (27). In accordance with this pathway and based on the fact that furin is enriched in the Golgi complex and acts as a protease for protein cleavage, a furin protease recognition site (RGRR amino acid sequence) was inserted between the GGSGG linker and Cj-CdtB. It is predicted that when the scFv + Cj-CdtB chimeric protein enters the Golgi, furin recognizes this site and breaks the peptide linker, which results in the scFv and Cj-CdtB separation and the CdtB segment could reach the nucleus alone (28). Weldon et al. designed immunotoxins based on Pseudomonas Exotoxin A that contained furin cleavage sites. Their study results showed the furin cleavage site is necessary for toxin activation in the Golgi apparatus and after scFv omission, the toxin can release to the cytoplasm (29). In accordance with the mentioned study results, in our immunotoxin, when scFv cleaved by furin protease in Golgi, the toxin could be transferred to the ER and then to the nucleus.
In the current study, the GORV web server was employed for the prediction of both scFv and scFv + Cj-CdtB secondary structures. This server estimates the probable secondary structures of proteins by predicting the impact of amino acids on the situations and structure of the adjacent amino acids. The comparison between the secondary structures of scFv and fusion protein, when Cj-CdtB attaches to scFv via the short linkers, showed no changes in their secondary structures. Based on the analysis of physicochemical features, the instability index for the fusion protein was 38.15, demonstrating that the scFv + Cj-CdtB protein is stable and its supposed high aliphatic index is associated with protein stability across a wide range of temperatures (30).
The I-TASSER web server was applied to create the 3D model of the scFv + Cj-CdtB recombinant protein based on the c-score. C-score is commonly within the range of -5 to +2, and more positivity signifies higher confidence of the model and vice versa (31). Among the 5 proposed 3D models constructed using this server for the chimeric protein, the model with the highest c-score was selected for further analysis. After the model structure’s refinement, the evaluation of unrefined and refined models was performed, using RAMPAGE and PROCHECK. Ramachandran plot results showed favor of residues residence before and after the model refinement. As illustrated in RAMPAGE results, most residues are determined in the favor and most favor regions, and even the following refinement, the favorness increased, showing that the models improved in the bond angles and location of amino acids. These results were confirmed by PROCHECK as well (Table 1).
The RNA Fold server was employed to predict mRNA secondary structures according to energy minimization. The data indicated that the free energy of the thermodynamic scFv + Cj-CdtB complex was -667.58 kcal/mol, indicating that the chimeric protein mRNA is stable. Since the chimeric construct contains a CdtB that is a part of a bacterial genotoxin, it may act as an allergen for the human body; therefore, the evaluation of its allergenicity potential is required. Allergenic elements elicit harsh responses from the immune system and may result in unfavorable allergenic effect morbidities (32). AlgPred web server predicted that the fusion protein is not an allergen for the human body. Ligand-receptor docking was used to investigate whether trastuzumab-derived scFv could retain its binding capacity to HER2 receptor and convey Cj-CdtB to HER2-positive breast cancer cells. Molecular docking was performed, using the ZDOCK server. The ZDOCK server results showed that scFv + Cj-CdtB chimeric protein can bind to HER2 receptors with high affinity and specificity. The visualization outputs determined that the binding ability of scFv + Cj-CdtB to its receptor was adequate.
5.1. Conclusions
The results reflected that scFv + Cj-CdtB could be a stable and non-allergenic chimeric protein with a proper affinity for increased HER2 receptors on breast cancer cells. Thus, the scFv + Cj-CdtB construct could be considered a new immunotoxin candidate against HER2-positive breast cancer.