1. Background
Axillary staging is one of the most important prognostic factors in breast cancer that plays a significant role in local and systemic treatment planning. The traditional method for axillary staging is axillary lymph node dissection (ALND). Nevertheless, there is some controversy about the effect of axillary dissection on the survival of patients with breast cancer. In a randomized trial with a long follow-up period, Fisher et al. showed that removing occult positive nodes at the time of the initial surgery has no significant survival advantage for patients who have undergone ALND (1).With this background, sentinel lymph node biopsy (SLNB) was selected as the standard method for the staging of the axilla in patients with clinically negative lymph node. The SLNB is a safe and accurate alternative to ALND with less morbidity, however about 7% false-negative rate has been reported for this procedure, and in these cases, axillary lymph node dissection may be omitted although it is necessary (2).
Although the physical examination is the first step in the evaluation of axillary LNs, most studies have reported a low sensitivity (8.3% - 41%), the specificity of 94.8% and, a negative predictive value (NPV) of 46.6% for this method (3, 4). Detecting whether a palpable lymph node is inflammatory, reactive or metastatic is difficult. The rate of false-positive results is about 53% in this method (5).
Axillary ultrasound (AUS) is a non-invasive, inexpensive, and available method that is routinely used in patients with breast cancer to determine the status of the LNs. Several studies have shown that AUS with or without fine-needle aspiration cytology (FNAC) is a suitable method for determining metastatic LNs with acceptable sensitivity and specificity.
According to studies, the diagnostic specificity of lymph node sampling under ultrasound guidance for core needle biopsy (CNB) or fine needle aspiration (FNA) might be as high as 100% (5).
The sensitivity of these methods is reported as 65% to 70% (6), and if positive, axillary dissection can be performed directly for the patient. These practices cannot be performed in all imaging centers and are highly dependent on the radiologists’ skill and available facilities.
2. Objectives
The aim of this study was to evaluate the sensitivity and specificity of axillary ultrasound with and without physical examination in the diagnosis of lymph node involvement in patients with breast cancer when there is no experience for sampling to confirm malignant involvement.
3. Methods
3.1. Patients
During a retrospective cross-sectional study, through a nonprobability convenience sampling, all patients who referred to the Motamed Cancer Institute (MCI) between Sep 2015 and August 2016 with a pathologic diagnosis of breast cancer were included. The ultrasound report and pathological diagnosis of the axillary LNs were recorded. The inclusion criteria consisted of patients with breast carcinoma, stage 0, I, II, and IIIA who had not received neoadjuvant chemotherapy.
Since the aim of this study was only to evaluate the sensitivity and specificity of AUS, without FNA or CNB, so those patients who had been subjected to these invasive procedures were excluded. Regarding the previous studies (7) which reported the sensitivity and specificity of AUS about 78% and 79%, respectively and α < 0.05 with the assumption of positive likelihood ratio (PLR) > 2.5, the predicted sample size was at least 100 patients. Informed consent was obtained routinely from patients for using data with considering their privacy. This study was accepted in the scientific committee and received the Ethics Committee approval. The work has been carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki).
3.2. Physical exam and Axillary Ultrasound
All patients underwent axillary palpation at Motamed Cancer Institute by two breast surgeons with more than ten years of experience in breast surgery. Positive PE is defined as clinically suspicious LN, it means a palpable firm or hard LN. The criteria for suspicious LNs by AUS were observing at least one of these morphological changes, including non-round shape, hypo echogenicity, cortical thickness > 2.5 mm, obliteration of the hilum and lobulation. Axillary ultrasound was performed by two radiologists who passed breast imaging course.
3.3. Axillary Surgery and Histological Exam
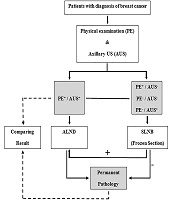
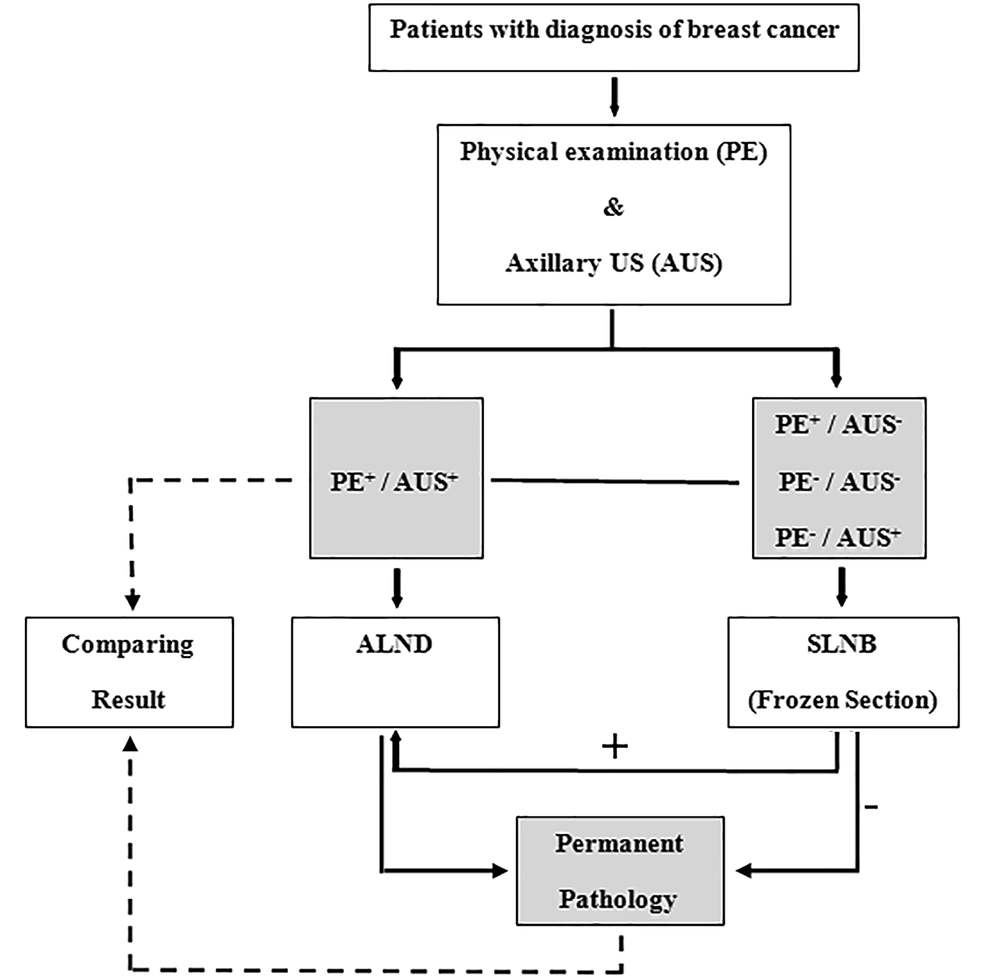
Axillary surgeries including SLNB or ALND were done based on the criteria, it means that ALND was selected in subjects with highly suspicious LN during palpation or AUS or in situations of positive SLNB. Before SLNB, lymphoscintigraphy was performed with a TC-99m with or without blue dye by sub-dermal, peri-areolar injection. Intraoperative detection of SLNs was carried out using a gamma probe and hot LNs, as well as the blue-colored LNs, were excised and evaluated by frozen section. The SLNs were evaluated using H&E (Hematoxylin and Eosin) staining. A metastasis ≥ 2 mm was taken to indicate macrometastasis and < 2 mm was taken as micrometastasis. Axillary lymph node dissection (ALND) was not performed in the cases with micro metastasis. The results were analyzed by comparing the PE and AUS results of LNs by permanent pathology (Figure 1).
3.4. Statistical Analysis
True positive (TP), true negative (TN), false positive (FP) and false negative (FN) of physical examination, AUS, and a combination of them were identified. The sensitivity [TP/(TP + FN)], specificity [TN/(TN + FP)], positive predictive value (PPV) [TP/(TP + FP)], and negative predictive value (NPV) [TN/(TN + FN)] were also calculated. The accuracy, positive and negative likelihood ratios (PLR, NLR) of AUS, PE, and combination of them were calculated through their specific formula. Statistical analysis was carried out by SPSS version 20, using the significance level of 0.05 for P value. The study was approved by the Ethic Committee of Motamed Cancer Institute (MCI) with code number IR.ACECR.IBCRC.REC.1394.5.
4. Results
A total of 140 patients were eligible for participation in the study. The mean age of the patients was 48.87 years (± 10.46) and their median age was 48 years (range: 25 - 81). The mean body mass index (BMI) was 27.55 (± 4.55) and 67.8% had a BMI ≥ 25 (range: 16 - 40).
The clinical and pathological characteristics of the subjects are presented in Table 1.
| Variable | Number (%) |
|---|---|
| Stage | |
| 0 (LCIS, DCIS) | 9 (6.4) |
| I | 48 (34.3) |
| II | 73 (52.1) |
| IIIA | 10 (7.1) |
| Tumor histology | |
| LCIS | 1 (0.7) |
| DCIS | 8 (5.9) |
| IDC | 117 (86) |
| ILC | 10 (7.4) |
| Tumor size | |
| T1 | 50(39.1) |
| T2 | 68 (53.1) |
| T3 | 10 (7.8) |
| Axillary surgery | |
| SLNB | 96 |
| ALND | 62 |
| Grade | |
| I | 6 (5.4) |
| II | 78 (69.6) |
| III | 28 (25) |
| ER/PR | |
| Positive | 104 (87.4) |
| Negative | 15 (12.6) |
| HER2 | |
| Positive | 47 (43.5) |
| Negative | 61 (56.5) |
| Breast surgery | |
| Mastectomy | 29 (21.8) |
| Lumpectomy | 104 (78.2) |
Abbreviations: ALND, axillary lymph node dissection; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LCIS, lobular carcinoma in situ; PR, progesterone receptor; SLNB, sentinel lymph node biopsy.
Among 62 patients who underwent ALND, 18 patients had positive sentinel node and 44 patients had suspicious LN in the physical exam. The flowchart of the management plan is shown in Figure 1.
Table 2 represents a summary of the AUS, PE, and combination of them compared to the pathology results. According to the table, the highest true positive was for combination of AUS and PE and the best true negative was related to PE.
| Pathology Results | Test Resulta | ||
|---|---|---|---|
| Positive | Negative | Total | |
| AUS | |||
| Positive | 30 (21.4) | 10 (7.1) | 40 (28.6) |
| Negative | 24 (17.1) | 76 (54.3) | 100 (71.4) |
| Total | 54 (38.6) | 86 (61.4) | 140 (100) |
| PE | |||
| Positive | 17 (12.1) | 2 (1.4) | 19 (13.6) |
| Negative | 37 (26.4) | 84 (60) | 121 (86.4) |
| Total | 54 (38.6) | 86 (61.4) | 140 (100) |
| Combination of PE and AUS | |||
| Positive | 38 (27.1) | 12 (8.6) | 50 (35.7) |
| Negative | 16 (11.5) | 74 (52.8) | 90 (64.3) |
| Total | 54 (38.6) | 86 (61.4) | 140 (100) |
Abbreviations: AUS, axillary ultrasound; PE, physical examination.
aValues are expressed as No. (%).
Table 3 shows the comparison of sensitivity, specificity, PPV, NPV, PLR, and negative likelihood ratio of AUS, PE, and AUS+PE. The highest sensitivity rate was related to AUS+PE with 70%. The best specificity was for AUS (about 88%) and the accuracy of AUS+PE was the highest (80%).
| Test | PE | 95% CI | AUS | 95% CI | AUS + PE | 95% CI |
|---|---|---|---|---|---|---|
| Sensitivity | 0.31 | 0.19 - 0.44 | 0.56 | 0.42 - 0.69 | 0.70 | 0.56 - 0.82 |
| Specificity | 0.98 | 0.94 - 1 | 0.88 | 0.82 - 0.95 | 0.86 | 0.77 - 0.93 |
| Positive predictive value | 0.89 | 0.76 - 1 | 0.75 | 0.61 - 0.88 | 0.76 | 0.65 - 0.85 |
| Negative predictive value | 0.69 | 0.61 - 0.78 | 0.76 | 0.68 - 0.84 | 0.82 | 0.75 - 0.88 |
| Positive likelihood ratio | 13.53 | 3.26 - 56.29 | 4.78 | 2.5 - 8.97 | 5.04 | 2.9 - 8.76 |
| Negative likelihood ratio | 0.7 | 0.58 - 0.84 | 0.5 | 0.37 - 0.68 | 0.34 | 0.23 - 0.52 |
| Accuracy | 0.72 | 0.64 - 0.79 | 0.76 | 0.68 - 0.83 | 0.80 | 0.72 - 0.86 |
Abbreviations: AUS, axillary ultrasound; PE, physical examination.
The accuracy of AUS was compared between the patients with BMI < 25 and > 25. The sensitivity of AUS was 60% for the patients with BMI < 25 and 52.9% for the patients who were overweight; the specificity was 83.3% and 86.48%, respectively (not shown in tables).
Among 53 patients with pathologically involved LNs, 36 (66.6%) had 1 - 2 (low-burden) and 18 (33.4%) had three or more (high-burden) involved LNs. Twenty-four patients had false-negative results by AUS, including six patients in the high-burden group (25%) and the rest (75%) in the low-burden group.
5. Discussion
The sensitivity of AUS in the diagnosis of axillary involvement was moderate (56%), its specificity was good (88%), and the accuracy was obtained 76% when lymph node morphology was used. The sensitivity increased up to 70% and when a classification of combined physical examination and AUS was used in comparison to each of them alone, the sensitivity decreased (86%).
Evaluation of axillary LN in breast cancer is crucial in determining the plan of treatment, including the type of breast and axillary surgery, immediate reconstruction, and the choice of systemic therapy. Inauspicious situations, the patient may become a candidate for neoadjuvant chemotherapy or directly undergone ALND without SLNB. If this probability is negligible, the patient becomes a candidate for SLNB and should be prepared in advance for the procedure (6).
What is the best method for determining the axillary LN status before surgery? Various studies have considered this challenge. Feng et al. showed that the sensitivity and specificity of axillary palpation are 32% and 95.5%, respectively (3). Other studies have reported a sensitivity of 8% - 35.5% and a specificity of 93% - 98.8% (7-9). The results of the present study showed a sensitivity and specificity of 31% and 98% for PE, which is consistent with previous findings. The false- positive rate for PE was 1.4% in this study, which is different from the results obtained by Specht et al., who estimated the rate as 23% in very suspicious cases and up to 53% in relatively suspicious cases. They reported overall false-negative rate of 25.7% for PE (5).
Imaging techniques can improve the diagnostic accuracy of physical examinations. Axillary ultrasound is one of the most common methods that is inexpensive and available, however largely dependent on radiologists’ experience. The sensitivity and specificity of ultrasound were reported as 63.8% and 73.6% by Gurleyik et al., 45% and 85% by Jackson et al., 54.3% and 100% by Gipponni et al., 58.6% and 89.4% by Feng et al., and 72% and 79% by Omranipour et al. (3, 10-12).
The ultrasonic criteria for LN involvement may be related to size or morphology. Previous studies which have used morphological criteria, reported AUS as an acceptable method for detecting involved LN. Alvarez et al. reported the sensitivity and specificity of the ultrasound to differentiate between benign and suspicious LNs as 54.7% - 92.3% and 80.4% - 97.1%., respectively. In the present study, the sensitivity was 56% and specificity was 88%, which is consistent with the results reported by Alvarez et al.
The only similar study in Iran was performed by Omranipour et al., who reported a sensitivity and specificity of 72% and 79%, respectively for AUS. In that study, the specificity of AUS was lower than ours. Like them, in some studies, only patients with normal physical examinations were included (10-12), while in the present study, the patients had both positive and negative examinations.
One of the most important reasons for the low sensitivity rate of the AUS in this study may be the selection of early-stage patients. Micrometastasis to the LNs is possible in these patients and may not lead to clear changes in morphology or tumor size in ultrasound.
The combination of PE and AUS improved sensitivity up to 70%, but the specificity did not change significantly (86% instead of 88%). In the case of positive PE and AUS, the probability of axillary involvement was 76% (38/50). The false negative rate of the combination test was 8.6% and these patients could be directly candidates for axillary dissection if FNA/CNB of LN is not accessible.
In 2011 with the publication of the results of the Z0011 trials, a new type of categorization was made for the first time which showed that patients with 1 - 2 sentinel lymph nodes with specific criteria (breast-conserving surgery, postoperative radiotherapy, and positive hormone receptors) should not undergo axillary dissection. In the present study, the false-negative AUS was reported in 24 patients, including six cases who had more than three (high-burden) and 18 who had 1 - 2 (low-burden) involvement. On the other words, in 75% of those who had false-negative axilla, axillary involvement was low-burden. It means that negative AUS predicts negative or low burden nodal involvement and these patients rarely have high-burden axillary involvement. Farshid et al. showed that, of those who had a false-negative AUS, LN involvement was low-burden in 86.8% (13). In another study, Jackson et al. showed the false-negative rate of AUS in detecting > 3 nodal involvement is 4% (12).
In most studies, sensitivity decreased, when FNA was added to the AUS, but specificity increased. Gipponi et al. (10) reported sensitivity as 44.1% and specificity as 100%, and they concluded that AUS alone might be suggested for early-stage breast cancer. FNA- positive findings are more reliant when there is a plan for neoadjuvant chemotherapy or ALND. Gurleyik et al. reported a 100% PPV for AUS+FNA (11).
Regarding the specificity of ultrasound and false-negative results, it is impossible to eliminate axillary surgery based on negative ultrasound results until the results of sound and INSEMA trials are released. Exclusion of axillary surgery for early-stage breast cancer may be possible in near future.
Although axillary palpation was performed by expert breast surgeons and axillary ultrasound by breast radiologists, we did not consider the difference between clinicians’ experience on the results and the lack of the reliability assessment could be a limitation of this study.
5.1. Conclusions
In conclusion, the axillary ultrasound is moderately sensitive with good specificity for diagnosis of lymph node involvement. The combination of physical examination and ultrasound could improve the sensitivity in comparing to each one alone. If both the physical examination and AUS are suspicious, axillary dissection could be considered when FNA or CNB of the lymph node is not available. Patients with negative axillary ultrasound and even the false-negative cases often have low-burden LN metastases and better prognosis even without ALND.