1. Introduction
Spindle cell carcinoma is an unusual form of squamous cell carcinoma that is specified by the World Health Organization (WHO) as a biphasic tumor including malignant mesenchymal and epithelial elements. This cancer shows an aggressive behavior and a poor prognosis. It is also known as pseudosarcoma, sarcomatoid carcinomas, and spindle or polypoid squamous cell carcinoma (1, 2). This tumor has been reported in various sites such as esophagus, upper aerodigestive tract, lung, gastrointestinal tract, breast, hepatobiliary system, and genitourinary system. The subsites of head and neck consist of salivary glands, pharynx, thyroid, larynx, thymus, oral cavity, and nasal area (2-4). Most of the cases are present in male patients between the 6th and 8th decade of life, with a clinical record associated with alcohol abuse, smoking, and radiation exposure (5).
2. Case Presentation
2.1. Clinical History
A 35-year-old female was referred to Alzahra Hospital, Isfahan in June 2015 with chief complaint of dental abscess. She had a painless swelling in the right side of maxilla that was appeared since past 6 months, but it was recently extended rapidly to orbit and nose. The medical history of the patient was unremarkable. The patient did not express any history of trauma in the maxillofacial complex, sinus-related disease, or use of alcohol and tobacco. An informed consent was taken from the patient.
Clinical examination revealed a swelling in the right side of her face from infraorbital to lower lip together with decreased mouth opening without any regional lymphadenopathy. Intraoral examination revealed halitosis, some teeth with deep decay, and polypoid mass, measuring 48 × 31 × 25 mm, extending from the right lateral incisor to the tuberosity with an ulcer in the center of lesion with the maximum dimension of 20 mm. The patient did not express any history of tenderness, dysesthesia, or paresthesia.
According to these clinical features, the primary clinical differential diagnoses were found to be benign inflammatory lesions such as pyogenic granuloma, peripheral giant cell granuloma, a locally aggressive infectious process, and a malignancy.
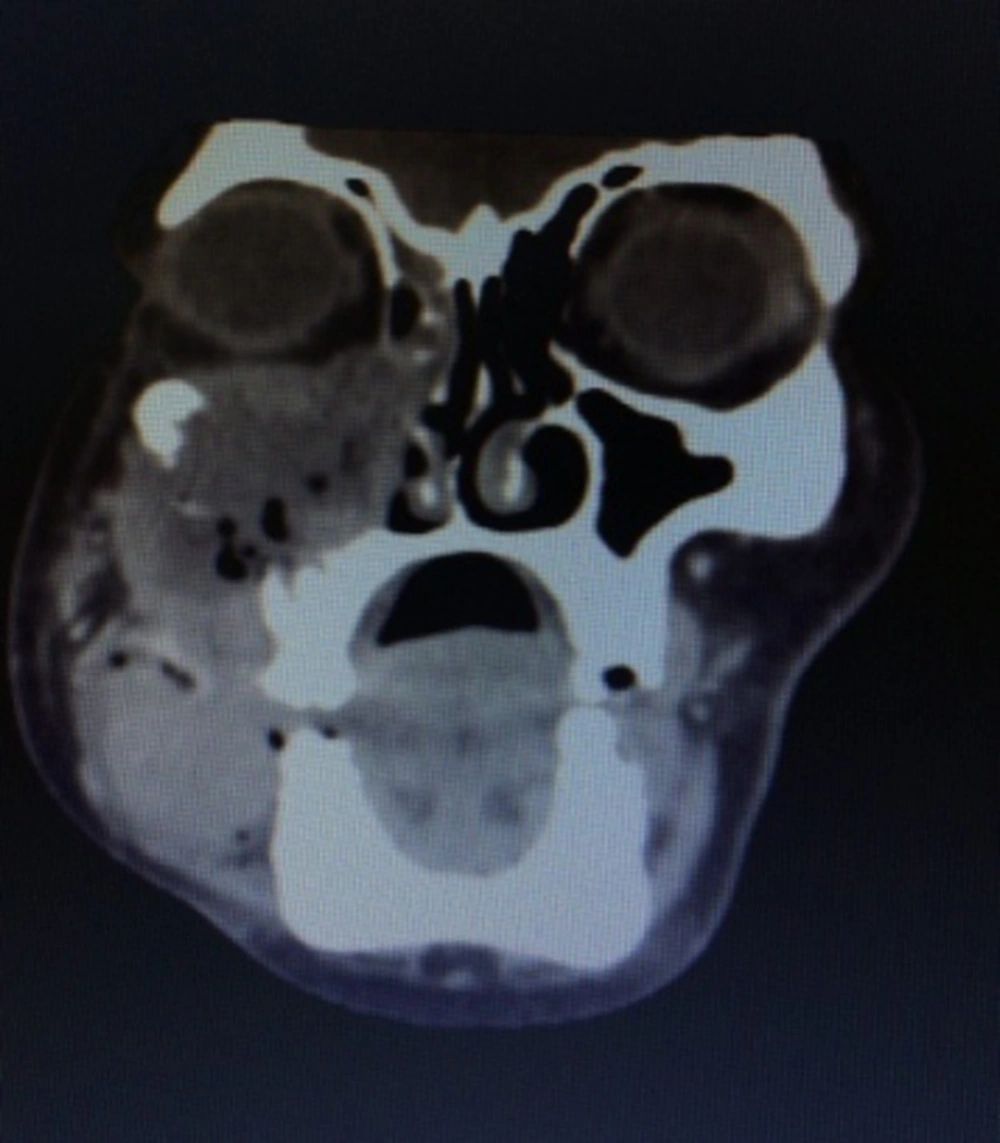
We also performed a CT scan on the patient. Her CT-scan results indicated a large destructive lesion with irregular margins that filled the right-side maxillary sinus, infiltrated to nasal cavity, lateral and inferior wall of orbit, masseter muscle, alveolar process, and internal region of low attenuation corresponding to necrotic areas. However, no lymph node and distant metastasis were detected (Figure 1).
Taking the radiographic appearance into consideration, our differential diagnoses included malignant aggressive lesions such as squamous cell carcinoma, lymphoma, soft tissue tumor (e.g. fibrosarcoma), and malignant tumors of minor salivary gland origin (e.g. mucoepidermoid carcinoma).
2.2. Diagnosis
The patient was admitted for additional tests. The vital signs, hematologic parameters, chest radiography, and bone scintigraphy were all normal and did not show any evidence of other lesions. An incisional biopsy was also performed from the oral aspect of the lesion.
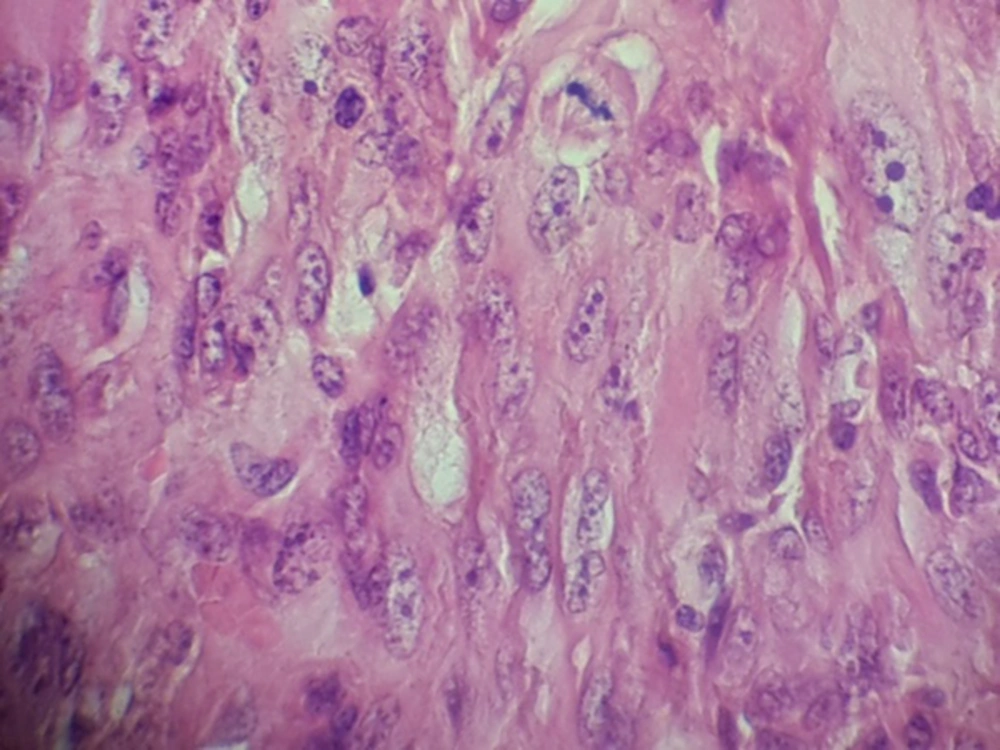
Hematoxylin and eosin–stained sections showed the proliferation of spindle cells in streaming and fascicular patterns, sometimes demonstrating epithelioid appearance with pleomorphism, hyperchromatism, and a lot of mitoses (Figure 2). According to these patterns, fibrosarcoma, leiomyosarcoma, melanoma, and carcinosarcoma were included in differentiated diagnosis and required immunohistochemical staining for CK, P63, EMA, S100, vimentin Ki67, CD99, Desmin, and Melan A.
Immunohistochemical staining of tumor cells showed focal positivity for CK and EMA, strong positivity for vimentin, 15% to 20% positivity for Ki67, and negativity for other markers. According to immunohistochemical findings, the lesion was diagnosed as spindle cell carcinoma.
2.3. Management
The patient was treated with a surgical process, consisting of right partial maxillectomy. Post-operatively, the patient underwent radiotherapy and chemotherapy on December 4th, 2015. There, she received 3-dimensional conformal radiotherapy (3-DCRT) and concurrent chemotherapy. The prescribed dose of radiotherapy was 68 Gy/30 fraction at 2GY/fraction, 5 fractions in 1 week. The area of radiotherapy included the tumor area, the right-side maxillary sinus, and chemotherapy treatment consisting of 50 mg/week cisplatin. After 5 months of primary treatment, the patient revealed recurrent tumor and pulmonary metastasis. She succumbed to the disease 12 months after the first diagnosis.
3. Discussion
Spindle cell carcinoma or sarcomatoid carcinoma is a rare carcinoma that can occur in many parts of the body such as the digestive and respiratory tract, breast, and kidney. Most sarcomatoid carcinomas in hollow viscus are polypoid and pedunculated (6, 7). The occurrence of this tumor in nasal cavity and paranasal sinus is rare (8), with only 15 cases reported in the English literature (4, 8, 9). The reported cases indicated male predilection with the age ranging from the third to seventh decade. Although tobacco, alcohol abuse, and previous irradiation have been considered as predisposing factors (3, 4), in our case, no habits were present. Some studies have reported that the spindle cell element of sarcomatoid carcinoma is a sign of metaplastic changes of the epithelial cells. This explanation has been supported by ultrastructural and histpathologic examinations (9, 10). Nappi et al. (11) indicated that epitheloid cell component is positive for keratin and negative for vimentin, while the spindle cell component is reactive for vimentin but nonreactive for keratin. We reported similar results except that the spindle cell component showed focal positivity for EMA and keratin, reflecting that the origin of these sacomatoid cells is epithelial carcinoma cells with true mesenchymal metaplasia.
There is a controversy about the management of spindle cell carcinoma due to the scarcity, difficulty of histological confirmation, and a shortage of literature about disease pathogenesis, outcome survey, and management guidelines (1, 3, 12).
Surgery seems to be the first option for primary and recurrent spindle cell carcinoma, where complete en bloc resection with larger safety border is needed. Either radiotherapy or chemotherapy is an alternative option for inoperable patients or those with sinonasal tumors. Overall, the time of diagnosis and the site of lesion affect the outcome so that early stage diagnosis and extraoral lesion have been resulted in better outcomes (12-14)). Some studies reported the increased mortality rate in the tumors of the head and neck area as a result of lower resistance to tumor-spreading along tissue planes and extensive lymph drainage system with resultant lymphatic and distant metastases (3, 4, 13).
Ellis et al. (15) reported a 36% survival rate in the patients with oral spindle cell carcinoma. Olsen et al. (16) reported 34 cases with laryngeal and hypopharyngeal spindle cell carcinoma; recurrence occurred in 10 cases and the 3-year survival rate was 56.8%. In Su et al.’s (17) series, the overall survival rate of oral spindle cell carcinoma was 27.5% over 3 years. The survival rate in our case was 12 months, which might be due to its late-stage patient.
4. Conclusions
It is necessary to expand the panel of immunohistochemistry staining for precise diagnosis of SpCC. In maxillary sinus, it shows rapidly growing with aggressive behavior and poor prognosis.