1. Background
Colorectal cancer (CRC) is the 3rd commonest cancer and the 4th commonest cause of cancer death globally. In Iran, the highest rates of CRC are respectively reported from provinces in the center, north, and west; however, CRC incidence rate varies among geographical regions (1). Genetically, CRC is a highly heterogeneous and multifactorial disease and family history, prolonged use of certain medications such as contraceptives, smoking, alcohol consumption, high-fat diet, chronic inflammation, insufficient intake of fruits and vegetables, high body mass index (BMI) values, and decreased physical activity are its risk factors (2). There are two types of CRC: hereditary CRC with an incidence rate of 5% - 10% and sporadic CRC with an incidence rate of 90% - 95% (3).
Nitric oxide (NO) is synthetized from L-arginine following nitric oxide synthase (NOS) activation by the stimulus, including hypoxia, shear stress, and cytokines (4). The impaired endothelial nitric oxide synthase (eNOS) activity can result in the clinical presentation of CRC and its mechanisms (5). The elevated NO level leads to DNA damage, prevention of DNA repair, as well as increase in tumor angiogenesis and metastasis (6). Neuronal NOS, inducible NOS, and eNOS are the isoforms of NOS introduced for mammals, which have 51% - 57% sequence homology with each other as well as different localization and inhibitor susceptibility (4). The NOS3 gene located on chromosome 7 (7q35-q36) encodes eNOS; it is a 21-kb gene consisting of 26 exons, which its expression can be regulated by stabilization, transcription, and phosphorylation. Although all three isoforms of NOS are expressed by endothelial cells, eNOS is the most frequent form including Glu/Asp (GT), Asp/Asp (TT), and Glu/Glu (GG) genotypes with alleles G and T (7, 8). The rs1799983 polymorphism (i.e., G894T) located in exon 7 formed by the transversion from G to T at codon 298 of eNOS (Glu298Asp) protein, which is known as one of the crucial polymorphisms of eNOS (9).
Glu298Asp polymorphism can reduce the activity of the eNOS gene and, subsequently, the NO levels in plasma. Asp298 variant can be effective in decreasing NOS activity (10, 11).
2. Objectives
Accordingly, the current study aimed at investigating the correlation of eNOS rs1799983 polymorphism (i.e., Glu298Asp, E298D, or G894T) with the risk of CRC in the Southwest of Iran.
3. Methods
In the present case-control research, 200 subjects were divided into two groups of patients with CRC and healthy controls (n = 100 in each group). All the patients and healthy controls were recruited from university hospitals in Ahvaz, Southwestern Iran from September 2016 to September 2017. The inclusion criteria for patients were the grade of tumor based on the surgeon’s opinion and positive histopathology report for CRC. Exclusion criteria for patients and controls were a history of other cancers, any experience of chemotherapy or radiotherapy, having inflammatory bowel disease, diabetes, thyroid disorders, and other inflammatory diseases. Subjects in the case and control groups were matched for age, gender, ethnicity, and race. Demographic and clinical characteristics, including gender, age, family history of cancer, alcohol consumption, and tobacco smoking were collected using a researcher-made questionnaire and medical records of patients. The subjects signed the informed consent form. The Declaration of Helsinki principles were observed in the study. The research ethics approval was obtained from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences.
3.1. eNOS G894T Polymorphism
Peripheral blood samples of the subjects were drawn into tubes containing EDTA and kept immediately at -20°C. To extract genomic DNA from leukocytes, the whole-blood technique (YektaTajhizAzma, Iran) as the gold standard was applied. DNA purity was assessed based on the absorbance ratio at 260 and 280 nm using a NanoDrop spectrophotometer. The PCR-RFLP was performed for eNOS G894T genotyping, using Ban II for digestion and 5’ TCC CTG AGG GCA TGA GGC T-3’ and 5’ TGA GGG TCA CAC AGG TTC CT-3’ as the forward and reverse primers, respectively described by Shin et al. (12). Polymerase chain reaction (PCR) was performed using a thermal cycler in a total volume of 25 µL. The PCR steps were as follows: 30 cycles of denaturation (2 min/95°C), annealing (45 sec/62°C), extension (1 min/72°C), and final extension (7 min/72°C). DNAs were digested with restriction enzymes in a 10-µL reaction volume containing 1 µL NE buffer, 1 µL PCR product, and 8 units of Ban II enzyme (Takara, Japan) for 24 hours at 37°C. Both digested and undigested amplicons were separated using a 2% agarose gel electrophoresis; to visualize the final products, an ultraviolet transilluminator was used.
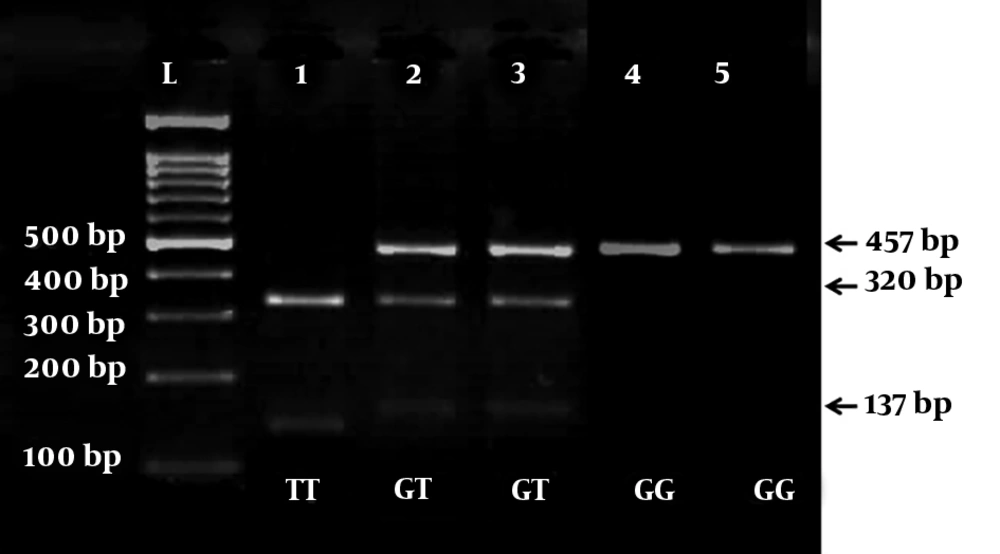
The PCR yield was divided into two fragments of 137- and 320-bp with G allele, whereas mutant allele T (457-bp) was not subjected to cleavage. Therefore, GT heterozygotes produced three 457-, 320-, and 137-bp fragments and wild-type homozygotes (GG) yielded two 137- and 320-bp fragments and mutant TT homozygotes showed one fragment of 457-bp (Figure 1).
3.2. Statistical Analysis
Data were analyzed using SPSS version 18. Student t was employed to compare continuous variables. The frequency of genotypes and alleles was assessed using the chi-squared test. If needed, odds ratios (ORs) were calculated with 95% confidence intervals (95% CIs) to evaluate the correlation of genotypes with CRC. To determine the independent risk factors for the TT genotype and T allele, logistic regression was employed. The level of significance was P < 0.05. Continuous data were expressed as mean ± standard deviation (SD).
4. Results
The clinical and demographic data of the study groups are shown in Table 1. In the case and control groups, the mean age of the subjects was 56.49 ± 13.89 and 58.15 ± 10.46 years, respectively. Both the study groups were matched by age, gender, and ethnicity; the groups had no significant difference (P = 0.34, 0.77, and 0.83, respectively). In addition, higher BMI value, family history of cancer, and habit of alcohol drinking were more prevalent in cases compared to controls (P = 0.027, P = 0.001, P = 0.001, respectively).
| Variable | Patient | Control | P Value |
|---|---|---|---|
| Total subjects | 100 | 100 | |
| Gender | 0.77 | ||
| Male | 49 (49) | 47 (47) | |
| Female | 51 (51) | 53 (53) | |
| Age, y | 56.49 ± 13.89 | 58.15 ± 10.46 | 0.34 |
| Smoking | 30 (30) | 31 (31) | 0.87 |
| Alcohol consumption | 11 (11) | 0 (0) | 0.001 |
| Family history | 19 (19) | 3 (3) | 0.001 |
| Anemia before diagnosis | 59 (59) | 0 (0) | 0.001 |
| Ethnicity | 0.837 | ||
| Lor | 62 (62) | 58 (58) | |
| Arab | 22 (22) | 25 (25) | |
| Other (Fars, Kurd, etc.) | 16 (16) | 17 (17) | |
| Parental marriage | 0.667 | ||
| Consanguineous | 40 (40) | 43 (43) | |
| Non-consanguineous | 60 (60) | 57 (57) | |
| BMI, kg/m2 | 0.027 | ||
| ≤ 18.5 | 12 (12) | 3 (3) | |
| 18.5 - 24.9 | 32 (32) | 27 (27) | |
| 25 - 29.9 | 39 (39) | 56 (56) | |
| ≥ 30 | 17 (17) | 14 (14) | |
| Clinical stage | |||
| I | 10 (10) | ||
| II | 38 (38) | ||
| III | 32 (32) | ||
| IV | 20 (20) | ||
| Site of tumor | |||
| Colon | 51 (51) | ||
| Rectum | 49 (49) | ||
| Metastasis | |||
| Yes | 6 (6) | ||
| No | 94 (94) |
Abbreviation: BMI, body mass index.
aValues are expressed as mean ± SD or No. (%).
According to data which are shown in Table 2, there was a significant difference between the case and control groups in terms of genotype distribution and allele frequency, based on the chi-squared test results (P < 0.05). The frequencies of the minor allele T and homozygous genotype (TT) in the CRC group was significantly higher than that of the control group (68% versus 32%, and 82.4% versus 17.6%, respectively). The ORs for TT and GT genotypes of G894T polymorphism were respectively 3.9 (95%CI = 1.8 - 3.6, P = 0.001) and 2.1 (95% CI = 0.6 - 2.8, P = 0.007) compared to the GG reference genotype. In addition, the adjusted ORs for TT and GT genotypes of G894T polymorphism were respectively 3.1 (95% CI = 1.6 - 3.2, P =0.001) and 1.8 (95% CI = 0.6 - 2.5, P = 0.005) compared to the GG reference genotype. The OR and adjusted OR of TT + GT genotype compared to the GG reference genotype were 2.9 (95% CI: 1.8 - 3.9; P < 0.005), and 3.1 (95% CI: 1.9 - 4.2, P < 0.005), respectively.
| Genotype/Alelle | Control Group (N = 100), No. (%) | CRC (N = 100), No. (%) | OR (95% CI) | P Value | AORa (95% CI) | P Valuea |
|---|---|---|---|---|---|---|
| TT | 6 (6) | 28 (28) | 3.9 (1.8 - 3.6) | 0.001 | 3.1 (1.6 - 3.2) | 0.001 |
| GT | 37 (37) | 44 (44) | 2.1 (0.6 - 2.8) | 0.007 | 1.8 (0.6 - 2.5) | 0.005 |
| GG | 57 (57) | 28 (28) | 1.0 (reference) | 1.0 (reference) | ||
| Dominant | ||||||
| TT | 6 (6) | 28 (28) | 3.2 (1.6 - 3.4) | 0.006 | 3.5 (1.9 - 3.8) | 0.006 |
| GT + GG | 94 (94) | 72 (72) | 1.0 (reference) | 1.0 (reference) | ||
| Recessive | ||||||
| TT + GT | 43 (43) | 72 (72) | 2.9 (1.8 - 3.9) | 0.006 | 3.1 (1.9 - 4.2) | 0.005 |
| GG | 57 (57) | 28 (28) | 1.0 (reference) | 1.0 (reference) | ||
| Alleles | ||||||
| T | 49 (24.5) | 100 (50) | 3.1 (2.0 - 4.7) | 0.001 | ||
| G | 151 (75.5) | 100 (50) | 1.0 (reference) |
Abbreviation: CRC, colorectal cancer.
aAORs were adjusted to age, gender, BMI, and stage of CRC.
5. Discussion
CRC, one of the commonest cancers, accounts for several neoplasm - related mortalities worldwide. Accordingly, several studies have been conducted on CRC to provide new insight into its etiology and also investigate the effects of environmental and genetic factors on the risk, onset, and course of the disease (13). NO is a critical signaling molecule associated with inflammation - mediated diseases such as cancer. Some studies showed an association of G894T, T - 786C, and 4b/a polymorphisms in eNOS with cancer; however, the findings are not contradictory (14). According to Fujita et al. (15), NO has anti - tumor activities, which sometimes plays a defensive role against cancer progression and metastasis. The eNOS - derived NO applies direct cytotoxic effects to cancer cells by inducing DNA damage and also has anti - tumorigenic effects (16). Some genetic studies compared healthy individuals with patients with cancer and revealed the association of NOS3 polymorphisms with some cancers, including CRC (17, 18). The missense eNOS - 7 Glu298Asp variant is responsible for transversion from Glu to Asp (in position 298) and increased risk of CRC in the Turkish population (11). Tumor progression can be promoted by the eNOS isoform as the main enzyme via the production of NO and several studies have focused on introducing new strategies to inhibit eNOS isoform (19, 20). Increased NOS expression is linked to many carcinomas and associated with tumor progression. The eNOS containing Asp at position 298 is easily cleaved and can decrease the production of basal NO (21). The current study assessed the correlation of the eNOS G894T polymorphism with CRC in 100 patients with CRC and 100 healthy subjects in the Southwest of Iran. Genotype or allelic frequencies of this polymorphism and CRC showed a significant correlation in the mentioned population. Arikan et al. (11) showed higher frequencies of G allele and GT genotype in patients with CRC than the healthy controls. The results of the present study showed that there is an association between G894T polymorphisms and the risk of colorectal cancer in the Iranian population. This study revealed that individuals bearing the T allele had a higher risk of colorectal cancer compared to those with the G allele. In the literature, findings of the NOS3 gene are controversial. Conde et al. (22) indicated that there is no correlation in the Spanish population between NOS3 Glu298Asp polymorphisms and colorectal cancer susceptibility. Yeh et al. (23) stated that in the Chinese population, individuals with the GG genotype of NOS3 G894 T polymorphism had an increased risk of colorectal cancer. Jang et al. (24) stated that the GT and TT genotypes of 894G > T polymorphism were associated with increased CRC susceptibility in the Korean population. Furthermore, we found that there was a significant correlation between the T allele of eNOS G894T polymorphism and CRC. A significant relationship was observed between a family history of cancer, drinking alcohol, higher BMI values, and cancer occurrence. This result was in line with those of previous studies in which alcohol use, a family history of cancer, and smoking were introduced as the CRC risk factors (25, 26). The eNOS G894T polymorphism has a significant relationship with CRC so that it can be attended as a predictor factor for CRC susceptibility.
5.1. Conclusions
According to the results of the present study, the T allele of eNOS G894T polymorphism and CRC were significantly correlated even after adjusting for traditional risk factors.