1. Background
According to the epidemiological studies, breast cancer is the most common cancer among women around the world (1). The incidence of this disease among Iranian women is increasing (2). Breast cancer is a heterogeneous disease and is divided into at least 4 main molecular subtypes: luminal A, luminal B, triple negative, and HER2- overexpressing tumors (3). These subtypes are different in terms of gene expression patterns, prognosis, and response to treatment. The luminal subtypes are ER/PR positive and show better prognosis than others subtypes. On the other hand, triple negative tumors do not express any of the hormone receptors and HER2. These tumors have very poor prognosis with limited treatment options. So, the identification of molecular markers in different subgroups of breast cancer may help choose specific treatment strategies in each subgroup in the future.
Cancer is characterized by alterations in gene expression profiles, which results in abnormal cell proliferation. EGFR (epidermal growth factor receptor) is a key player in proliferation, angiogenesis, and metastasis (4). So, it may play a fundamental role in the pathogenesis of various malignancies. The dysregulation of this gene is taken into consideration in multiple tumor types, including breast cancers (5-8). The assessment of EGFR expression in malignancies is important due to the availability of anti-EGFR drugs that were approved by FDA such as erlotinib and gefitinib. These drugs inhibit the tyrosine kinase domain of EGFR protein. Clinical trials are underway in order to investigate the effects of these drugs on patients with breast cancer (9, 10).
The results of previous investigations demonstrated that EGFR expression in breast malignancies show differences among various subtypes and ethnic groups (11). According to the previous studies, EGFR expression was significantly increased in Asian patients compared with Caucasians patients (11). Thus, anti-EGFR therapies depend on detailed information about EGFR expression status in patients with breast cancer. It should be noted that the relationship between EGFR mRNA expression and clinicopathological features has rarely been reported in breast cancer.
2. Objectives
In this study, we aimed at evaluating the status of EGFR expression at the transcriptional level in luminal (as a good prognosis subtype) and triple negative (as a poor prognosis subtype) breast tumors in comparison to normal tissues in Iranian patients. Also, we investigated the association between the mRNA expression of this gene with clinicopathological characteristics of patients with breast cancer.
3. Methods
3.1. Patients and Tissue Collection
In this study, were obtained 52 tumor specimens from patients with primary breast cancer. The studied samples included 27 luminal tumors, 25 triple negative tumors, and 6 normal breast tissues (cosmetic mamoplasty specimens). All tissues were obtained from the Iran national tumor bank (INTB) of the Cancer Institute of Iran in Imam Khomeini Hospital. Written informed consent was obtained from all patients; this study was approved by the local ethical committee at Tehran University of Medical Sciences. None of the participants were under chemo or radiotherapy before surgery. the clinicopathological features of the patients were collected from their medical records in INTB. These features included age, tumor size, ER, PR and HER2 status (based on immunohistochemistry results), axillary lymph node involvement, grade, and stage (Table 1).
| Characteristics | Number in Luminal Group | Number in Triple- Negative Group |
|---|---|---|
| Age | ||
| < 50 | 17 (63) | 13 (52) |
| ≥ 50 | 10 (37) | 12 (48) |
| Tumor size | ||
| ≤ 2 | 2 (7.4) | 1 (4) |
| 2 - 5 | 22 (81.5) | 21 (84) |
| > 5 | 3 (11.1) | 3 (12) |
| Grade | ||
| I | 8 (29.6) | - |
| II | 14 (51.8) | 3 (12) |
| III | 5 (18.6) | 22 (88) |
| Stage | ||
| I | - | - |
| II | 21 (77.8) | 24 (96) |
| III | 6 (22.2) | 1 (4) |
| Nodal status | ||
| Positive | 16 (59.3) | 3 (12) |
| Negative | 11 (40.7) | 22 (88) |
| HER-2 | ||
| Positive | 4 (14.8) | - |
| Negative | 23 (85.2) | - |
| Total | 27 | 25 |
aValues are expressed as No. (%).
3.2. Extraction of the Total RNA from Tissues and cDNA Synthesis
Hybrid-RTM kit from GeneAll company (Korea, Cat.No: 305-101) was applied to extract RNA according to the manufacturer’s instructions. The ratio of absorbance at 260 nm and 280 nm (A260/280) was used to assess RNA purity and quantity by spectrophotometer.
First-strand cDNA was generated by HyperscriptTM kit from GeneAll company (Korea, Cat.No: 601-005) according to the manufacturer’s instructions.
3.3. Real Time PCR
Real-time PCR assay was accomplished, using Rotor-Gene 6,000 cycler (Corbett Life Science, USA). PUM1 (Pumilio RNA binding family member 1) was used as the housekeeping gene for normalizing data. Selected primer sequences included EGFR forward 5´-AGGCACGAGTAACAAGCTCAC-3´, EGFR reverse 5´-ATGAGGACATAACCAGCCACC-3´; PUM1 forward 5´-AGTGGGGGACTAGGCGTTAG-3´, and PUM1 reverse 5´-GTTTTCATCACTGTCTGCATCC-3´.
To prepare the reaction mix for real-time PCR we added 0.5 μL forward and 0.5 μL reverse primers (primer concentration: 5 pmol), 10 μL RealQ Plus 2 × Master Mix Green (AMPLIQON, Denmark, cat.No: A323499), 1 μL target cDNA, and 8 μL sterile water in a total volume of 20 μL. Reaction conditions: 95°C for 15 minutes, followed by 40 cycles of 95°C for 15 seconds and then, 59°C for 60 seconds. Samples were analyzed in duplicate and average threshold cycle (CT) values were applied. We used normal breast tissues as calibrator to obtain the relative threshold cycle (ΔCt), and the relative expression between breast tumors and normal breast tissues was calculated, using the 2-ΔΔCT method (12).
3.4. Statistical Analysis
SPSS 21 statistical software was used for data processing. The data were presented with mean and 95% CI for numerical data or frequency and percentage for qualitative data. Student’s t test was used to assess the mean difference in the expression of BRCA1 or EGFR between luminal and triple negative groups. The Pearson correlation statistic was used to evaluate the linear correlation between EGFR expression and tumor size. The linear regression model was applied to evaluate the linear effect of EGFR expression on tumor size. The association between the expression of EGFR with clinicopathologic factors (age, axillary lymph node metastasis, grade, stage, ER, PR and HER2 status) were analyzed by t test or one-way analysis of variance (ANOVA) tests and alternative non-parametric tests. A P value less than 0.05 was considered as statistically significant.
4. Results
4.1. Study Groups
The relative expression of EGFR gene was assessed in 52 breast cancer specimens, including 27 luminal and 25 triple negative tumors. The mean age of the studied population was 48 years old, ranging from 29 to 81. The clinicopathological characteristics of patients in luminal and triple negative subtypes are summarized in Table 1.
4.2. Expression Status of EGFR mRNA in Breast Tumors
As the expression of EGFR mRNA values for all 6 normal breast samples were between 0.32 to 2.66, values 2.8 or more were considered overexpression status and values 0.2 or less were considered underexpression status in breast tumors. We have shown the frequency of different statuses of EGFR mRNA expression in luminal and triple negative tumors in Table 2.
| EGFR mRNA | HR+/HER2+ or HER2- (n = 27) | HR-/HER2- (n = 25) | Total (n = 53) |
|---|---|---|---|
| Expression level median (range) | 0.14 (0.01 - 1.26) | 0.83 (0.04 - 5.94) | 0.35 (0.01 - 5.94) |
| Expression status | |||
| Underexpression, % | 59.3 | 8 | 34.6 |
| Normal expression, % | 40.7 | 80 | 59.6 |
| Overexpression, % | 0 | 12 | 5.8 |
4.3. Differences in Expression Patterns of EGFR mRNA in Triple Negative and Luminal Subtypes
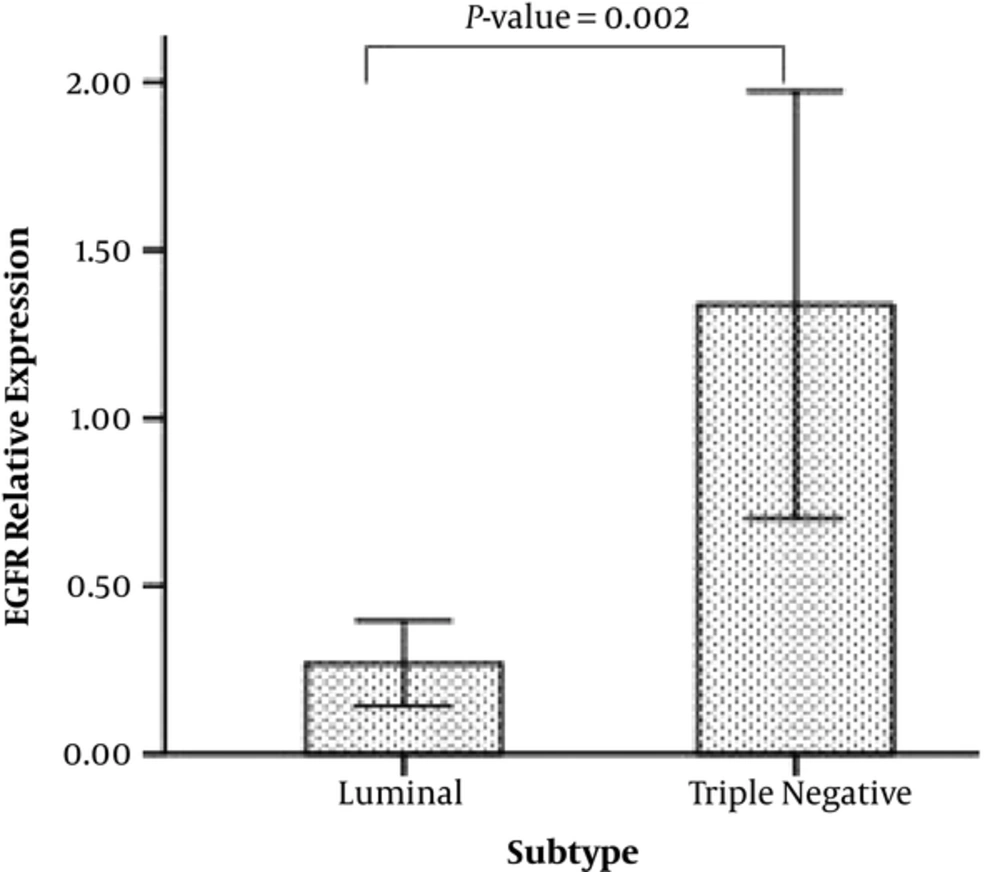
An independent samples t test was conducted to compare the EGFR relative expression for triple negative and luminal tumors. This analysis showed that the levels of EGFR mRNA in triple negative group (Mean = 1.3, SD = 1.5) were higher than luminal group (Mean = 0.26, SD = 0.32). This difference was statistically significant (P = 0. 002) (Figure 1).
4.4. Association Between EGFR Expression and Clinicopathological Characteristics of Patients
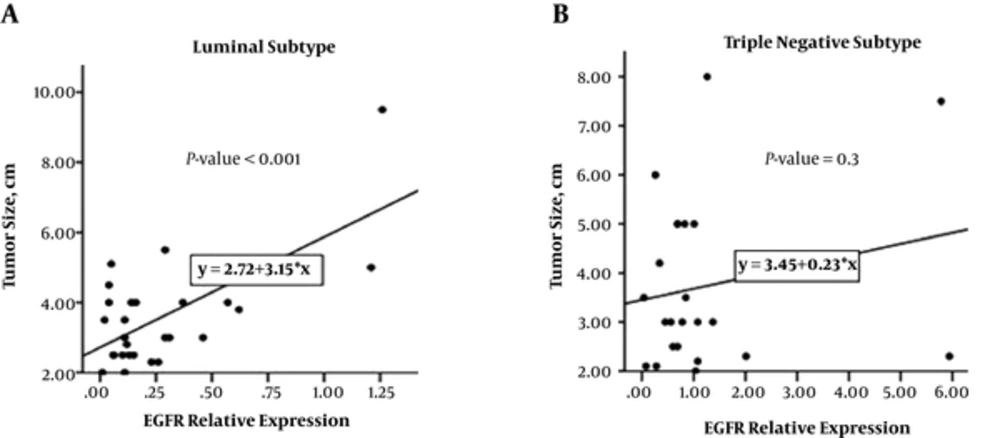
The results of the present study showed that there is a positive correlation between gene expression and size of luminal tumors (r = 0.662, P < 0.001), but this correlation was not seen in triple-negative tumors (P = 0.3) (Figure 2). The linear regression model demonstrated that for every 1 unit additional EGFR relative expression in luminal tumors, we would expect to see 3.15 unit additional size of tumor (Figure 2A).
Also, independent Samples t test in 52 samples revealed that the increased expression of EGFR was significantly associated with ER negative (P = 0. 001), and PR negative (P = 0.001) tumors. Mann-Whitney test displayed that the increased expression of EGFR shows a trend in HER2 negative tumors, which did not reach to the statistically significant level (P = 0.052). Kruskal-Wallis Test determined that the increased expression of EGFR was significantly associated with grade III in breast tumors (P = 0.017). In this study, EGFR mRNA expression did not significantly associate with age at diagnosis, lymph node status, and stage of the tumors.
5. Discussion
EGFR has an important role in the control of complex cellular events such as cell growth, angiogenesis and metastasis (4). EGFR dysregulation can lead to EGFR pathway activation during several malignancies (5, 7, 8). The results of the previous studies demonstrated that EGFR expression in breast cancer has shown heterogeneity among different subtypes and ethnic groups (11). Thus, EGFR-targeting treatments will need detailed information about EGFR expression status in patients with different pathological features. The current study demonstrated that EGFR expression shows a discrepancy between different subtypes of breast tumors in Iranian patients, confirming some previous studies (13). The results of this study showed that EGFR mRNA was overexpressed only in triple negative tumors (12%) and none of the luminal tumors displayed EGFR overexpression. On the other hand, there is a high frequency of EGFR underexpression in luminal breast tumors (59.3%) compared to triple negative tumors (8%). Anti-EGFR therapies would be discriminablely indicated for patients with breast cancer with normal or overexpressed EGFR. Accordingly, the results of the current study suggest that approximately 92% of patients with triple negative tumors (80% normal expression and 12% overespression) are likely to benefit from anti-EGFR therapies on the basis of EGFR mRNA expression status.
In this study, we demonstrated that EGFR mRNA expression in triple negative tumors shows a significant increase compared to luminal tumors. This result are in line with the previous studies evaluating EGFR protein (14, 15).
The previous studies about the association between EGFR protein expression and clinicopathological characteristics are summarized in Table 3. Significant association between EGFR expression and large tumor size has been reported in several studies on breast cancer tumors, while studies that assessed only triple negative tumors did not observe this association. We investigated the additive effect of EGFR expression on tumor size in triple negative and luminal tumors, separately. Surprisingly, this analysis showed that increase in EGFR expression is associated with the enlargement of luminal tumors, but not in triple negative tumors. This result suggests that EGFR expression has an interaction effect on tumor size. Hence, this effect may depend on the tumor subtype. This observation could justify the inconsistency between previous studies (Table 3). Because in studies that assessed all subtypes of breast tumors probably major portion of sample size consist of luminal tumors. Accordingly, this subtype can predominantly exert influence on the final conclusion of these studies. We speculate that the increased expression of EGFR alone in triple negative tumors might not be enough for the enhancement of tumor size, and additional molecular alterations might be necessary.
| Study | Patients | Technique | Results |
|---|---|---|---|
| Sainsbury et al. 1985 (16) | 108 BC | LBA | EGFR expression with ER-negative, high grade and size > 5 cm |
| Sainsbury et al. 1987 (17) | 135 BC | LBA | EGFR expression with ER-negative, high grade and size > 5 cm |
| Harris et al. 1989 (5) | 220 BC | IHC | EGFR expression with ER-negative |
| Tsutsui et al. 2002 (18) | 241 recurrent BC | IHC | EGFR expression with ER-negative |
| Buchholz et al. 2005 (19) | 82 BC | IHC | Not significant |
| Viale et al. 2009 (20) | 284 TN | IHC | Not significant |
| Magkou et al. 2008 (21) | 154 BC | IHC | EGFR expression with ER negative, high grade |
| Nicholson et al. 2010 (22) | 2567 BC | LBA | EGFR expression with ER/PR negative, HER2 positive, young-age (< 50), size > 2 |
| Nozoe et al. 2011 (23) | 37 BC | IHC | EGFR expression with ER/PR/HER2 negative, high grade |
| Nakajima et al. 2014 (24) | 84 TN | IHC | Not significant |
| Park et al. 2014 (6) | 151 TN | IHC | Not significant |
| Sobande et al. 2015 (25) | 52 TN | IHC | Not significant |
| Present study | 27 Luminal and 25 TN | Real time PCR | EGFR expression associated with ER/PR negative, high grade tumors and tumor size increase only in luminal tumors |
Abbreviations: BC, breast cancer; IHC, immunohistochemistry; LBA, ligand binding assay; TN, triple negative.
In the present study, the results showed that the increased expression of EGFR mRNA is significantly associated with ER/PR/HER2 negative status and grade III in breast tumors. As shown in Table 3, the significant association between EGFR protein expression with ER-negative status and high histological grade in breast tumors have been seen in several studies. So, in this context, according to previous investigations, it seems that EGFR expression at mRNA and protein levels are generally consistent. Finding an association between EGFR expression and ER-negative tumors in several literatures demonstrates a cross-talk between the EGFR and ER signaling pathways. Although, a number of studies did not find any association between EGFR expression and hormone receptors (6, 19, 20, 24, 25). This contradiction may be due to the difference in study population. Most of these studies have examined only triple-negative breast tumors (Table 3).
In conclusion, EGFR expression shows a discrepancy between different breast tumor subtypes. It seems the majority of patients with triple negative tumors may be eligible to receive anti-EGFR therapies. Clinical trials in the future should monitor the response to EGFR inhibitors in triple negative patients with overexpressed and normal expressed EGFR.