1. Background
Postoperative radiotherapy significantly decreases local recurrence rates in breast cancer; it prevents 1 death out of 4 recurrences of breast cancer (1). The basis for the utilization of partial breast radiation therapy in the place of whole-breast irradiation depends on the result that almost 85% to 90% of breast recurrences are limited to the same quadrant of the breast as the primary tumor, typically under 1 cm from the initial lumpectomy site (2). Most of the papers in radiation therapy have suggested delivering multiple consecutive small doses (50 Gy delivered in fractions of 2 Gy/day), which control tumors with low normal tissue complications. Recent and rapidly evolving knowledge of the radiobiology and the alpha/beta ratios of different body tissues and tumors suggest that some tumors, such as breast cancer, can favorably respond to the single-fraction high dose of radiation (3, 4). Intraoperative radiotherapy (IORT) is a creative apparatus to convey the ideal portion of radiotherapy, following the excision of cancer to the margins of resection. Additionally, the effects of IORT may likely extend beyond the direct DNA breakage, such as its influence on the resultant tissue microenvironment and immune system (5).
Two great prospective randomized trials compared IORT with conventional whole breast radiation therapy (WBRT) (6, 7). The first study is TARGIT-A, a prospective randomized trial; it was conducted between 2000 and 2012, in which 3451 patients received IORT versus standard WBRT. The trial used the Intrabeam (Carl Zeiss, Oberkochen, Germany) low-kV energy x-ray (7, 8). They found that in the IORT group, 15.2% of the patients subsequently required WBRT due to the final pathology, showing high-risk characteristics such as positive lymph nodes, positive surgical margins, or high-risk tumor biology such as invasive lobular cancer. The IORT was subsequently served as the boost dose (TARGIT-B) (6). Receiving IORT and then WBRT in these cases showed that it decreases local recurrence (9) and no increase was observed in toxicity or complications with the addition of WBRT to IORT (10). The 5-year reported in breast relapse level for all women treated in this study (TARGIT-A) was 3.3% for IORT versus 1.3% for WBRT (P = 0.042). After evaluating the patients, who received radiation during surgery instead of a delayed process, the results showed that the risk of local recurrence was 2.1%, which was not statistically different from patients receiving WBRT (P = 0.31) (6).
The second study is ELIOT (external radiotherapy for early breast cancer); it is a prospective randomized trial conducted between 2000 and 2007 (7). They enrolled 1305 patients that received 21 Gy high-voltage electron IORT (IOERT), utilizing LIAC linear accelerators (Sordina IOERT technologies SPA; Italy). The 5-year local recurrence was 4.4% vs. 1.4% in external beam radiation therapy (EBRT) (11), but while considering criteria for low-risk tumors, they were found to be 1.5% in 5-year and in subset analysis, the survival was the same. IORT boost combination with whole breast irradiation (WBI) is presently examined in two multicenter prospective trials; low-kV-IORT in the multicenter TARGIT-B study with 20 centers and 1800 patients and IOERT in Hypofractionated whole-breast irradiation (HIOB) trials. HIOB trial following intraoperative electron boost reduced the whole treatment period without compromising local control rates; the multicenter HIOB trial began in January 2011 as an International Society of Intraoperative Radiation Therapy (ISIORT). In that study, boost IOERT of 10 Gy is blended with hypofractionated WBI (15 × 2.7 Gy) for breast cancers (stages I/II). A comparable idea of IOERT, in addition to short-term WBRT, was tried in a stage II design by the Milano group (12).
The advantages of IORT are alleviating psychological distress, allowing an earlier return to normal life, and reducing related expenses, including the cost of the procedure and other indirectly associated expenses (13-15).
IORT has less fibrosis and radiation toxicity compared to whole breast radiation. It also spares the other healthy tissues, such as heart, lung, and the remainder of the breast tissue from unnecessary radiation exposure and complications (16).
Studies have shown inconsistencies between radiation oncologists in arranging cavity boosts and variety in targets of above 1 cm (17). Precisely targeting therapy to the lumpectomy cavity with IORT directs radiation only to the site that needs radiation and a 1-cm margin spares the other healthy surrounding tissue from unnecessary radiation. The studies suggested that giving IORT as a boost for high-risk patients, followed by planned WBRT, may further decrease their risk of local recurrence by improving both temporal and geographical miss (18-20).
The direct visualization of the radiation device in the lumpectomy cavity avoids the geographical miss of an incorrectly directed boost of standard radiotherapy because of a lack of clips or seroma to accurately define the lumpectomy cavity. Also, IORT can safely be combined with oncoplastic breast conserving surgery (BCS), which allows the radiation to be accurately directed to the lumpectomy cavity. Studies have shown no increased wound-healing complications associated with this combined procedure (21, 22).
Moreover, radiation to the skin is decreased by shielding and it might be associated with improved cosmesis and the possible elimination of radiation-induced angiosarcoma. Relapse levels are negligible. Longer follow-up and studies are currently tested to determine true efficacy, but theoretically, IORT, as a replacement for the boost dose, is reasonable and may even be more accurate since it is directed during the initial surgery (19).
It is anticipated that the use of IORT will continue to increase as further data accumulate in ongoing studies regarding its long-term efficacy.
2. Objectives
The aim of this study was to investigate the intraoperative boost radiotherapy with 50 kV X-rays versus external radiotherapy in breast cancer.
3. Methods
3.1. Sample
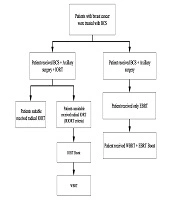
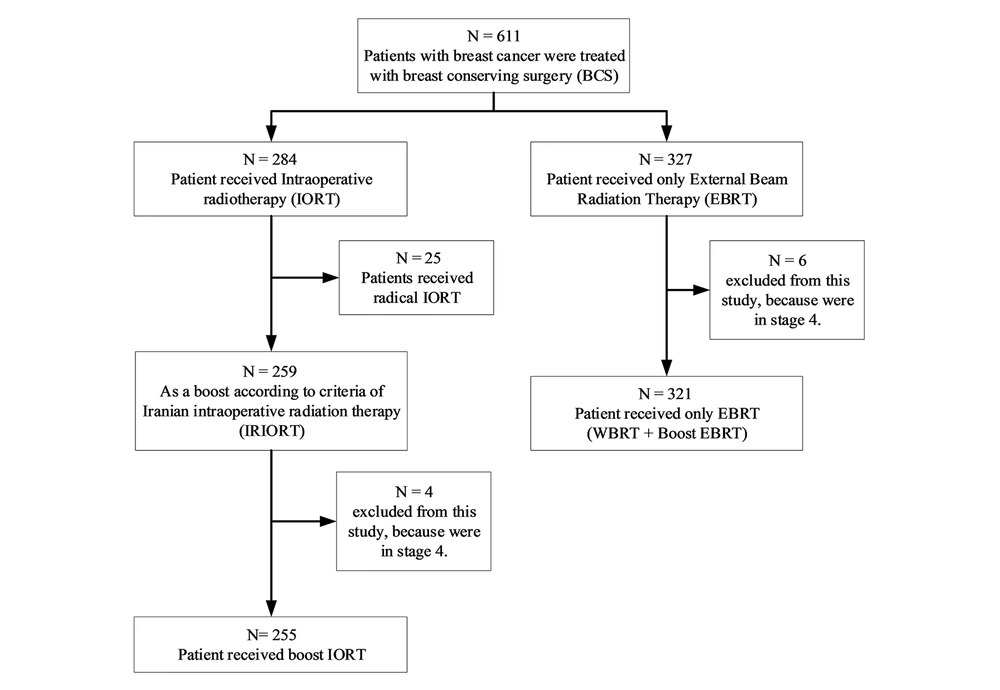
Totally, 611 patients with breast cancer were treated with BCS in the Cancer Research Center of Shahid Beheshti University of Medical Sciences (April 2014-September 2018). Generally, 327 patients received only EBRT; 284 patients received IORT (50 kV energy X-rays, with 20 Gy). Then, 25 patients received radical IORT and 259 as a boost according to criteria of Iranian intraoperative radiation therapy (IRIORT) protocol (Table 1); 4 patients of the IORT group and 6 patients of the EBRT group were in stage 4 (with bone metastasis). So, they were excluded from this study. Figure 1 shows the inclusions of each group.
| Factors | Appropriate | Possible | Contraindicated |
|---|---|---|---|
| Age, y | ≥ 45 | 40 - 44 | < 40 |
| Tumor size, mm | < 30 | 30 - 35 | > 35 |
| Margin | Negative | Negative | Positive |
| Nodal status | Negative | Negative (i-, i+) | Positive |
| IDC | Yes | Yes | - |
| ILC | Yes | Yes | - |
| Pure DCIS | ≤ 30 mm | 30-40 mm | > 40 mm |
| Grade | 1 or 2 | Any | - |
| LVI | Negative | Any | - |
| ER, PR | Positive | Any | |
| HER2 | Any | - | - |
| EIC | < 25% | ≥ 25% | Diffuse |
| LCIS associated | Any | Any | Any |
| Multifocality | No | Yes | - |
| Multicentrity | No | No | Yes |
| Axillary surgery | SLNB | SLNB or ALND | - |
| Neoadjuvant | Not allowed | Not allowed | If used |
Iranian Intraoperative Radiation Therapy (IRIORT) Agreements for Radical IORT
3.2. General Description
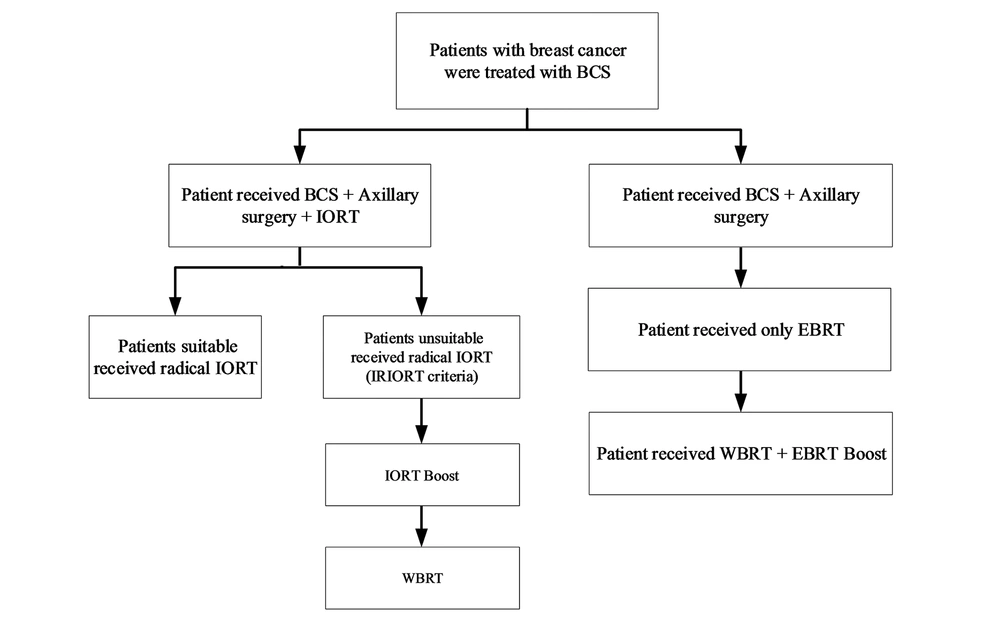
Regarding the patients with documents of breast cancer in core needle biopsy, wide local excision and sentinel node biopsy (SLNB), or axillary dissection (if the node were clinically positive) were done. When specimen margins were reported free by frozen section pathologist, the lumpectomy cavity was measured and a spherical applicator was selected based on the cavity size (2.5 - 5 cm) by consult with radio-oncologist and physicist in IORT team. The applicator and radiation source were, then, placed into the lumpectomy cavity as targeted intra-operative radiotherapy (TARGIT-A trial) study (12). Next, meticulous hemostasis was confirmed. Purse-string suture was placed to approximate the posterior and anterior breast parenchyma around the applicator for achieving compliance of the cavity with the applicator. Care was taken to make certain that all breast tissues in the cavity appose and no parts of the skin were under 1 cm from the applicator. The radiation-oncology and physicist team (23) used computer-calculated dosimetry in order to control the time of the radiation needed to deliver 20 Gy to the applicator surface; it was calculated based on the size of the spherical applicator used (18 - 51 min). After the surgery, according to the permanent pathology report, patients with age ≥ 45, node free, and tumor size < 30 mm were categorized as a suitable group (Table 1) and did not receive EBRT. In addition, some patients in the possible group received IORT as a radical according to multiple favorable conditions (25 patients radical). The rest of the patients received IORT as a boost dose and they received WBRT after the surgery (255 patient’s boosts). In Figure 2, we explained the strata based on the type of treatment for each group.
3.3. Statistical Analysis
The overall survival and disease-free survival (DFS) determined the interval time between diagnosis and the last follow-up or event. The primary outcome was the occurrence of recurrence (local and systemic) and death. Local recurrence includes an ipsilateral breast or axilla. In this study, we compared recurrence after tumor bed boost with IORT (low-energy X-rays) and EBRT for 54 months. Cumulative hazard function and survival plots were illustrated by the Kaplan-Meier method. The “log-rank” test was used to evaluate the survival variance between the two treatments. In the final step of the analysis, we used Cox’s multivariate proportional hazard model to compare the DFS between two groups with adjusting covariates, including stages (stage 1 - 3), estrogen receptor (ER), or progesterone receptor (PR) status (positive or negative), and menopause status (pre-menopause and post-menopause). The data were analyzed by SPSS 24.
The Ethics Committee of Cancer Research Center, Shahid Beheshti University of Medical Sciences approved this study.
4. Results
In this study, 255 patients with a boost dose of IORT were compared with 321 patients with EBRT. All breast surgeries were performed by a single surgery team and IORT was done by a single physicist and radiation-oncology team. Generally, 39 patients (15%) in the IORT group and 41 patients (13%) in the EBRT group received neoadjuvant chemotherapy due to local advance diseases. All of them received radical whole breast EBRT, including 50 Gy in 25 fractions. The control group received EBRT followed by a boost dose of 10 Gy in the other 5 fractions. The patients visited their physicians every 6 months.
In addition, out of 25 patients in the radical dose IORT group, there was only 1 local recurrence (4%). In this patient, the primary tumor was high-grade ductal carcinoma in situ (DCIS), estrogen receptor (ER) negative, and the size was 60 × 50 × 40 mm. She was in the contraindicated group for radical IORT and was advised to receive EBRT after the surgery according to the protocol (Table 1), but she did not follow. Local recurrence occurred with invasive tumor 26 months after the surgery. All the patients received 50 Gy whole breast radiations in 25 to 28 fractions after the surgery. In 4 metastatic (stage 4) patients in the IORT group, no event was seen in mean 425 days follow-up. In 6 metastatic patients in the control group, 1 death occurred 180 days after the surgery. All patients with stage 4 were excluded from the study.
The mean ± SD age of the patients was 46.76 ± 11 and 49 ± 11 years for the IORT and EBRT groups, respectively, and they had a significant difference (P = 0.331). The most histology of tumors was invasive ductal carcinoma (92.5% for the IORT group vs. 91.5% for the EBRT group). Invasive lobular carcinoma was 2.8% in both groups and pure DCIS was 3.5% in the IORT group vs. 4.1% in the EBRT group. Tumors were most commonly hormone receptor-positive (71.9% in the IORT group vs. 75.7% in the EBRT group), human epidermal growth factor 2 (HER2) receptor non-amplified (75.9% in IORT vs. 75.8% in EBRT), and Ki67 < 20% (38.5% in the IORT group vs. 41.9% in the EBRT). Most women had tumor grades 2 and 3 with no lymphovascular invasion (LVI) (IORT = 53.7% vs. EBRT = 71.9%). Most patients were in stage 2 in both groups. In the IORT group, 4.8%, 57.8%, and 27.5% were reported for stages 1, 2, and 3, respectively. In the EBRT group, 21.9%, 49.8%, and 28.2% were reported for stages 1, 2, and 3, respectively. Most tumors were < 30 mm, but patients in the IORT group had greater tumor size (mean size of 32.5 mm in the IORT group vs. 27.3 mm in the EBRT group)
| Patients Factors | IORT | EBRT | P Value |
|---|---|---|---|
| Ageb | 0.331 | ||
| < 40 | 76 (29.8) | 83 (26.3) | |
| 40 – 45 | 24 (9.4) | 41 (12.9) | |
| ≥ 45 | 155 (60.8) | 192 (60.8) | |
| Menopausec | 0.000 | ||
| Pre-menopause | 136 (63.8) | 136 (47.4) | |
| Post-menopause | 77 (36.2) | 151 (52.6) | |
| Sized | 0.009 | ||
| < 30 | 142 (59.2) | 203 (69.3) | |
| 31 - 35 | 18 (7.5) | 27 (9.2) | |
| > 35 | 80 (33.3) | 63 (21.5) | |
| Gradee | 0.029 | ||
| 1 | 34 (14.6) | 26 (9.1) | |
| 2 | 95 (40.8) | 147 (51.0) | |
| 3 | 104 (44.6) | 115 (39.9) | |
| LVIf | < 0.001 | ||
| Negative | 124 (53.7) | 192 (71.9) | |
| Positive | 107 (46.3) | 75 (28.1) | |
| ER, PRg | 0.333 | ||
| Positive | 151 (71.9) | 231 (75.7) | |
| Negative | 59 (28.1) | 74 (24.3) | |
| HER2h | 0.982 | ||
| Negative | 176 (75.9) | 172 (75.8) | |
| Positive | 56 (24.1) | 55 (24.2) | |
| Antigen KI-67i, % | 0.542 | ||
| < 20 | 60 (38.5%) | 62 (41.9) | |
| > 20 | 96 (61.5) | 86 (58.1) | |
| Histologyj | 0.962 | ||
| IDC | 235 (92.5) | 290 (91.5) | |
| ILC | 7 (2.8) | 9 (2.8) | |
| IDC + ILC | 3 (1.2) | 5 (1.6) | |
| DCIS | 9 (3.5) | 13 (4.1) | |
| EIC | |||
| Positive | 36 (14.1) | 60 (19.9) | |
| Stagek | 0.065 | ||
| 1 | 27 (11.5) | 70 (21.9) | |
| 2 | 141 (60.0) | 159 (49.8) | |
| 3 | 67 (28.5) | 90 (28.2) | |
| Lymph nodel | 0.148 | ||
| Positive | 121 (47.5) | 168 (41.4) | |
| Negative | 134 (52.5) | 188 (58.6) |
Clinical, Pathologic, and Biologic Characteristics for IORT (N = 255) and EBRT (N = 321) Groupsa
The most used applicator sizes were 35 mm, 40 mm, and 50 mm. Most recurrences occurred while using 40 and 50 applicators.
There were no statistically significant differences between IORT patients and EBRT patients for local recurrence, systemic recurrence (metastasis), any recurrence, and death (P > 0.13). There were 3 patients (1.2%) with local recurrence in IORT patients compared to 8 patients (2.5%) in EBRT patients. The number of deaths attributable to cancer was 8 patients (3.1%) in the IORT group compared with 10 patients (3.1%) in the EBRT group, showing no significant difference. The 1-, 2-, and 5-year DFS were 99.9%, 93.3%, and 85.1% in the IORT group, respectively; these rates were 96.9%, 94.3%, and 86.0% in the EBRT group, respectively. Table 4 shows the descriptive statistics for comparing recurrences, death, and DFS in the two groups.
| IORT | EBRT | P Value | |
|---|---|---|---|
| Local recurrence | 3 (1.2) | 8 (2.5) | 0.133 |
| Systemic recurrence (metastasis) | 12 (4.7) | 20 (6.2) | 0.805 |
| Any recurrence | 15 (5.9) | 22 (6.9) | 0.637 |
| Death | 8 (301) | 10 (3.1) | 0.988 |
| DFS/1 year | 99.9% (2 events) | 96.9% (9 events) | |
| DFS/2 years | 93.3% (10 events) | 94.3% (14 events) | |
| DFS/5 years | 85.1% (15 events) | 86% (22 events) |
Local, Systemic Recurrence, and Death in the IORT and EBRT Groupsa
Most tumors metastasis were in the brain (5), liver (4), bone (4), lung (2), and multifocal (3) (Table 5).
| Total | Local | Systemic | Bone | Lung | Liver | Brain | Multifocal |
|---|---|---|---|---|---|---|---|
| 15 | 3 | 12 | 4 | 2 | 4 | 5 | 3 |
Site of Recurrences in the IORT Group
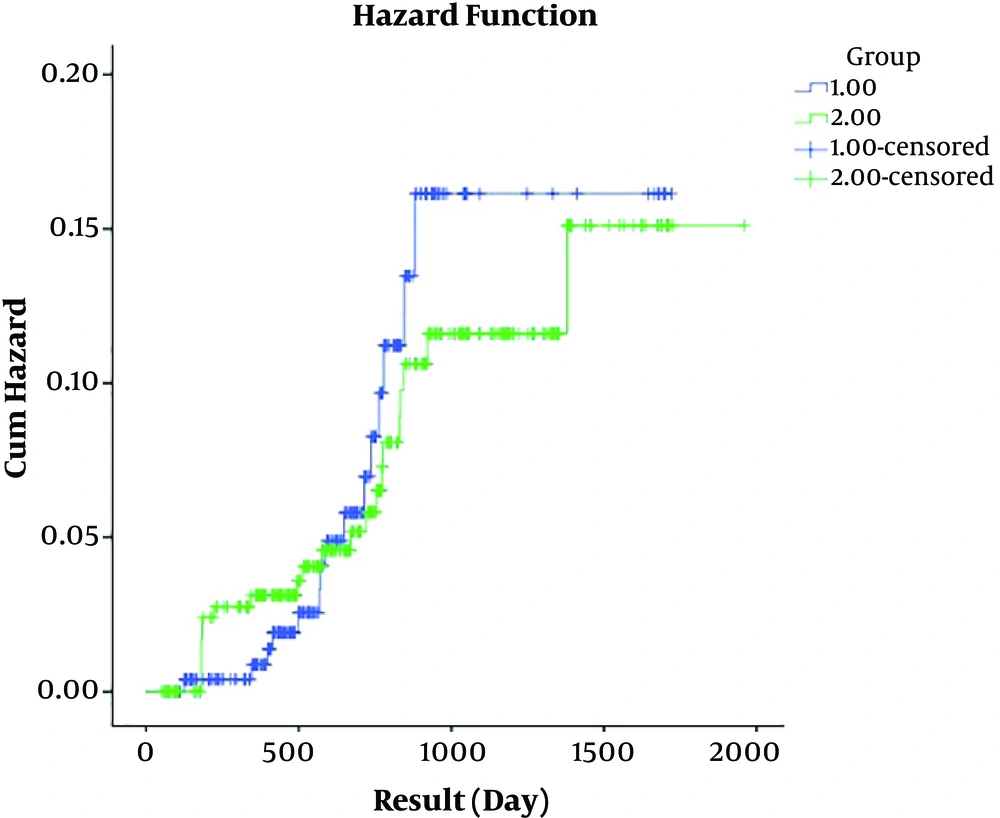
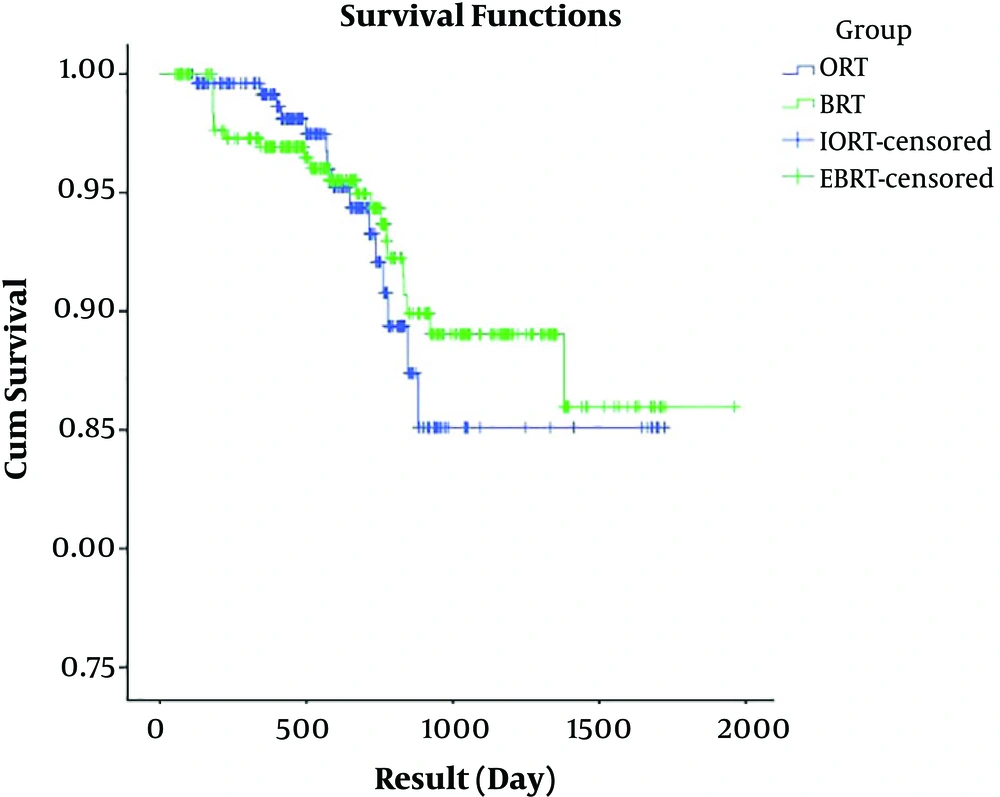
The mean ± SD of DFS was 1567.76 ± 39.97 and 1793.85 ± 35.60 days for the IORT and EBRT groups, respectively. The result of the log-rank test showed no significant difference between mean DFS days in the two groups (P = 0.715). Regarding Figures 3 and 4, it is concluded that patients in both groups had rather similar DFS and hazard ratio (HR) in terms of the event under the study, however, we found no significant difference between the two groups in terms of DFS (HR = 1.043; P = 0.948).
5. Discussion
To the best of our knowledge, this study was conducted for the first time to evaluate the consequences of patients with breast cancer treated with low-kV X-ray IORT in Iran. In this retrospective study, the patients with breast cancer, who received a boost dose of IORT with X-ray, showed similar DFS to EBRT boost dose. Local recurrence was 1.2% vs. 2.5% and systemic recurrence was 4.7% vs. 6.2%, respectively. All 15 patients with recurrences were invasive ductal carcinoma; 10 patients were stage 3 and 5 patients were stage 2. All of them were node-positive. Four patients with systemic recurrence received neoadjuvant chemotherapy due to locally advanced disease and others received adjuvant; 5 patients were ER, PR positive, 9 patients were LVI positive, and 5 patients were HER2 positive. There were more aggressive tumors in younger women. While considering these variables together, HR became non-significant (1.043). In metastatic (stage 4) patients that were excluded from this study, no event occurred in the IORT group in 425 days, but 1 death occurred on the 180th day after the surgery in the EBRT group.
Based on the pathological analysis, the maximum density of the tumor cell was approximately 90% of microscopic remainders that were detected in 4 cm nearby the edge of the macroscopic tumor (24, 25). Up to 80% of tumor relapses were seen in the former index quadrant (1-26). After delivering a boost dose to the tumor bed following the WBRT, the retrospective analysis reported lower relapse levels. Dose escalation to an electron boost of 10 Gy to 16 Gy (5 – 8 × 2 Gy) or alternatively interstitial implants (HDR-brachytherapy) was verified in large randomized prospective trials and local relapse levels were split (27, 28). The impact can be seen in all age groups; however, the absolute gain was mostly seen in the ages under 45 years (28). A chief benefit of intraoperative boost radiotherapy is the close nearness of the walls of the surgical cavity in the absence of postoperative hematoseroma and delivering optimal dose of radiotherapy just after the excision of cancer during the same operation to the well-vascularized tissue and to the margin of resected cancer to damage all the cancer cells remaining nearby the tumor just on time of surgery (29).
The aim of IORT while utilizing a low-kV x-ray set is to attain the utmost irradiation of the tumor cavity to a 1- to 2-cm tissue depth and it means decreasing the residual invasive tumor foci to below 5% (24-30). The TCD50 (the range that locally controls 50% of adult solid tumor) for the microscopic residual disease is closer to 25 Gy to 50 Gy (31). The IORT boost of 20 Gy, 15 Gy, or 10 Gy preceded or followed by 45 Gy fractionated EBRT may have a theoretical biological impact of 95 Gy, 76 Gy, or 61 Gy supposing an α/β of 10 (32).
The current investigations reveal that low-kV X-ray might generate a microenvironment, which is not proper for invasion or growth of a tumor (5). DNA strand breaking is the chief mechanism producing cell death due to radiation, but investigations imply the radiobiological effect of non-DNA-related mechanisms (33). Single-large dose RT induces anti-tumor immune responses, intervening the regression of non-irradiated tumors, or metastases distant from the irradiation site, a process known as the “abscopal effect” (34). In 2003, Camphausen et al. (35) reported TP53 as an important mediator and recognized a radiation-dose dependence to induce this effect (36) as the “distant bystander effect” and out-of-field systemic anti-tumor effects (23). A mechanism may be related to the depletion of regulatory T cells (T reg) and myeloid-derived suppressor cells that limit the function and proliferation of autoimmune cells (37). Cell membrane damage by radiation and lengthy antigen exposure is another older hypothesis. The autoimmune effect of radiation is dose-dependent and seems to need a large fraction size. It is tumor histology-independent and may be amplified by suitable systemic or locally-administered cytokines that function as an immune adjuvant (38). By changing the tumor into an in situ vaccine, radiotherapy might activate host immune mechanisms and immunize a patient against cancer.
5.1. Conclusions
We found that IORT, as a tumor bed boost with a 50 kV x-ray in breast conserving therapy, had a better outcome, but it was not significant. It had at least no inferiority compared with EBRT. More investigations with more sample size and an extended follow-up period are recommended.
5.2. Limitations
One of the limitations of this study was the follow-up period.