1. Background
Although thyroid cancer is a rare type of cancer worldwide, which is considered to be the most common endocrine malignancy with an estimated incidence rate of 1.7% (1,2). Thyroid cancer frequently affects women in diverse geographical areas (1). In the process of thyroid cancer, the estrogen receptors may be developed and proliferated (3). According to the reports of the National Cancer Institute (NCI), the increase in the prevalence rate of thyroid cancer has been more significant compared to the other cancer types (4).
The main causes of thyroid cancer remain unclear, while various risk factors have been determined for the disease, including exposure to UV radiation, inheritance, age, and dietary iodine concentration (3). Thyroid cancers with the highest prevalence are anaplastic thyroid cancer, follicular thyroid cancer, and papillary thyroid cancer (PTC), while PTC has the highest incidence among all the other types (5-7).
Recently, oxidative stress has been shown to be involved in the pathogenesis of numerous cancers and the associated complications. After several steps, free radicals are inevitably generated from the normal metabolic reactions of the microsomal membrane, chains of mitochondrial electron transport, and auto-oxidation. Damage to the cell membrane structure and function is imposed by hydroxyl radical, through which lipid peroxidation is initiated, leading to the production of malondialdehyde (MDA) (8). Therefore, serum MDA measurement is used as a marker for the oxidative damage caused by lipid peroxidation in tissues.
Peroxidation rate is determined based on the balance between the generation and elimination of lipid peroxides. Imbalance between the removal and generation of lipid peroxide may be resulted from decreased cell defense or increased peroxidation (9). These reactions are terminated by removing the free radical mediators and preventing oxidative reactions through the antioxidative defense mechanism of the body caused by their auto-oxidative properties. This defensive mechanism involves the enzymatic and non-enzymatic systems. In the case of an enzymatic reaction, enzymes such as SOD, GSH-Px, CAT, and GST interfere with the removal of the free radicals. A non-enzymatic reaction system mainly involves the binding of amino acids, vitamins, and proteins to metals such as VitC, VitE, carotene, Cys, Met, His, Ser, Glucose, transferrin, lactoferrin, ceruloplasmin, and glutathione (GSH).
Among antioxidants, GSH is the most abundant intracellular tripeptide consisting of the sulfhydryl (SH) group that actively removes potentially harmful electrophiles and metals, which could produce toxic oxygen radicals (8). Thyroxine synthesis by the thyroid gland may lead to the inflammation and active proliferation of a tumor and ultimately oxidative stress (9). Reports have indicated that increased free radicals and decreased antioxidant status in the patients with thyroid cancers may lead to oxidative stress (10).
Recent studies focusing on saliva as a sample for cardiovascular diseases, autoimmune disorders, cancers, and oral conditions have confirmed its diagnostic potential, attracting the attention of researchers as an investigative specimen (11).
The present study aimed to measure the levels of MDA and total antioxidant capacity (TAC) in the serum and saliva of PTC patients. Furthermore, we evaluated the diagnostic use of saliva as a noninvasive specimen to compare the antioxidant status in the patients with thyroid cancer and healthy subjects.
2. Methods
This case-control study (age- and gender-matched) was conducted at the research institute for endocrine sciences in collaboration with Shahid Beheshti University of Medical Sciences (school of allied medical sciences, department of laboratory sciences) in Tehran, Iran during 2014 - 2016.
2.1. Ethical Considerations
The present study was performed in compliance with the Helsinki declaration and ethical principles in research, and the study protocol was approved by the medical review board of Shahid Beheshti University of Medical Sciences. Informed consent was obtained from the participants, and they were assured of anonymity and confidentiality terms regarding their information. In addition, the research objectives and details were disclosed.
2.2. Subjects
Study population included 33 PTC patients with the mean age of 34.6 ± 8.02 years, who referred to Taleghani Hospital in Tehran, Iran with their diagnosis based on the clinical-pathological examination after performing total thyroidectomy and before radioiodine therapy. Additionally, 33 non-relative healthy volunteers were enrolled in the study as control subjects, who were matched in terms of age and gender and had no history of thyroid diseases. Participants with a history of severe, chronic cardiovascular, pulmonary, and renal diseases were excluded from the study. Demographic forms were completed by the selected subjects.
2.3. Samples
2.3.1. Blood Samples
Blood samples (3 mL) were collected from the participants. After the coagulation of the samples and centrifugation at 3000 rpm for 10 minutes, the obtained sera were stored at the temperature of -80°C to be assayed in the following stages.
2.3.2. Saliva Samples
To collect the saliva samples, the participants were instructed not to brush their teeth within 4 - 5 hours prior to sample collection. Following that, they were asked to thoroughly rinse their mouths with water and remove food particles or contaminants. After five minutes, saliva (3 mL) was collected without prior stimulation. After the centrifugation of the samples at 3000 rpm for 10 minutes, the supernatants were stored at the temperature of -80°C until assayed.
2.4. TAC Assay
Measurements of the serum and saliva TAC levels were performed using a commercial kit (ZellBioGmbH, Germany) in accordance with the instructions of the manufacturer for the colorimetric assay of oxidation reduction at the wavelength of 490 nm. TAC levels were regarded as the antioxidant contents in the samples as opposed to the action of ascorbic acid as the standard measure. Through this method, TAC could be determined with the sensitivity of 0.1 mM (100 μmol/L). The variation coefficients of the intra- and inter-assays were specified to be less than 3.4% and 4.2%, respectively.
2.5. MDA Assay
MDA level was measured using a commercial colorimetric assay kit (MDA assay kit, ZellBio GmbH, Ulm, Germany) in accordance with the instructions of the manufacturer. In this method, the MDA associated with a high temperature reacts with thiobarbituric acid, so that further MDA could be colorimetrically measured on an acidic medium at the temperature of 90 - 100°C at 535 nm. MDA could be determined with the sensitivity of 0.1 μM. The variation coefficients of the intra- and inter-assays were specified to be 5.8% and 7.6%, respectively.
2.6. Statistical Analysis
Data analysis was performed in MedCalc software version 14.8.1, and data were expressed as mean ± standard deviation (SD). Normality of the data was assessed using the Kolmogorov-Smirnov test, and Mann-Whitney U test was used in the case of non-normal distribution. Moreover, independent t-test was applied to compare the mean values between the case and control groups. In all the statistical analyses, P value of less than 0.05 was considered significant.
3. Results
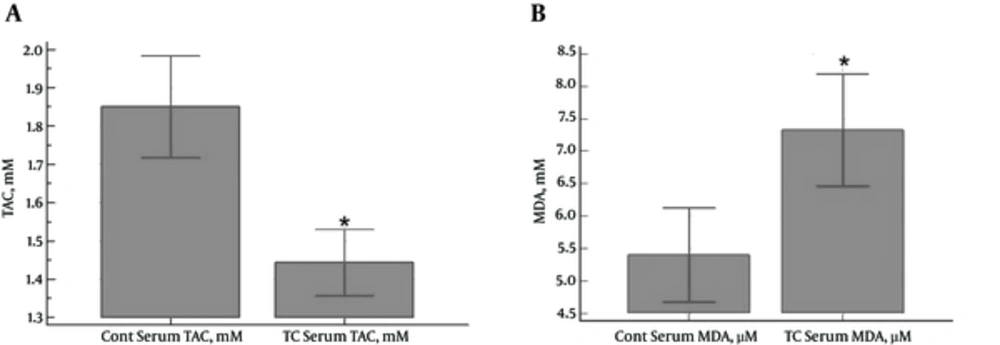
In total, 33 PTC patients and 33 healthy subjects were enrolled in the study. No significant differences were observed between the case and control groups in terms of age and gender distribution. According to the results, serum TAC levels were significantly higher in the control group compared to the PTC patients (1.85 ± 0.37 and 1.44 ± 0.24 mM, respectively) (P < 0.0001) (Figure 1A). In contrast, the PTC patients had significantly higher serum MDA levels compared to the control group (7.33 ± 2.43 and 5.40 ± 2.05 µM, respectively) (P = 0.0009) (Figure 1B).
A, Serum Levels of TAC (mM) in PTC Patients and Control Subjects (a Significant Reduction in the Case Group Compared to the Control Group) (P < 0.0001);B, Serum Levels of MDA (μM) in PTC Patients and Control Subjects (a Significant Increase in the Case Group Compared to the Control Group) (P = 0.0009)
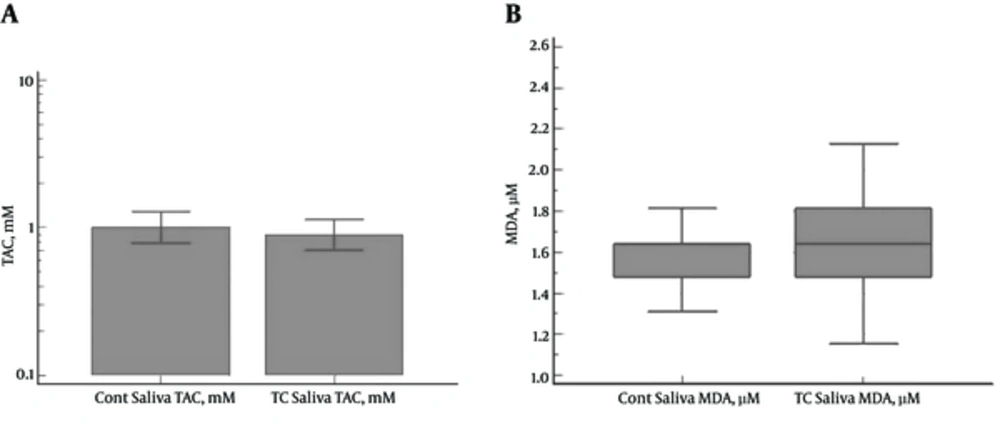
No significant differences were denoted between the PTC patients and control subjects in the salivary TAC (1.10 ± 1.01 and 1.14±0.74 mM, respectively) (P = 0.4839) and MDA levels (1.74 ± 0.47 and 1.56 ± 0.19 µM, respectively) (P = 0.2598) (Figure 2A and 2B).
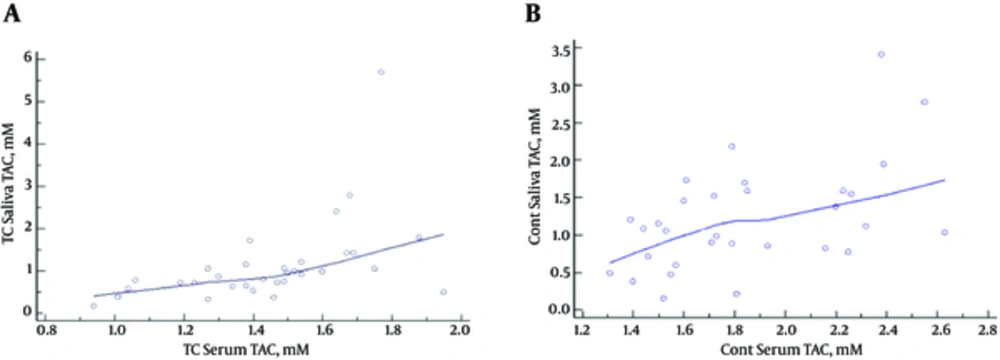
According to the findings, there was a significant correlation between the serum and salivary TAC levels in the PTC patients (r = 0.4861; P = 0.0048) (Figure 3A) and control subjects (r =-0.514; P = 0.0031) (Figure 3B). On the other hand, no significant association was observed between the salivary and serum MDA levels in the case group (r = 0.055; P = 0.7) and control subjects (r = -0.05; P = 0.7).
4. Discussion
The free radicals generated from the metabolic reactions in the body are removed by antioxidants. Disruption of the equilibrium between the generation and removal of free radicals leads to oxidative stress and changes in the macromolecules, such as nucleic acid, lipids, and proteins (12). Currently, mounting evidence suggests that free radicals play a pivotal role in tissue transformation into malignancy (13,14).
Human cancer is mainly developed by free radicals, causing changes in the DNA base through breaking the strands, damaging the tumor suppressor genes, and excessively expressing the proto-oncogenes (12). Although all cell components are sensitive to free radicals, lipids seem to be affected most significantly in the process. MDA is an end product of lipid peroxidation. Oxidative damage to cells and tissues may be indicated by the serum level of MDA, marking lipid peroxidation (8). Evidently, free radicals are involved in the physiological and pathological processes in the thyroid gland (13,14). According to previous studies, thyroid cancer is associated with enhanced oxidative stress (15,16).
In the present research, we measured the serum TAC and MDA levels in PTC patients and healthy controls, observing the TAC levels to be significantly lower in the PTC patients compared to the control subjects, while the serum MDA levels were significantly higher in the PTC patients compared to the control group.
Moreover, the salivary TAC and MDA levels were measured in the case and control groups, indicating no significant differences in this regard. However, the salivary concentrations of TAC and MDA in the PTC patients were respectively lower and higher compared to the control subjects. Consistent with our findings, Sener et al. reported significantly lower levels of TAC in breast cancer patients compared to the control group (17). Furthermore, Hosseini-Zijoud et al. demonstrated significantly lower TAC concentrations in the patients medullary thyroid cancer compared to healthy controls, which could be due to the consumption of antioxidants as a result of augmented metabolism and production of free radicals (8).
In another study, Totan et al. stated that serum and salivary TAC levels were significantly lower in the patients with oral lichen planus (OLP) compared to the controls; nevertheless, the obtained results suggested that the TAC level alone cannot thoroughly demonstrate the antioxidant status in various phases of the disease, and therefore, it does not provide a reliable estimation for the previous or future ulcers. On the other hand, serum and salivary levels of GPx and uric acid could be used as more accurate parameters for antioxidant capability (18).
According to the results obtained by Vlkova et al. patients with oral premalignant lesions had significantly lower salivary TAC levels, which could be the cause of the higher oxidative stress markers (19). In addition, Miricescu et al. have reported that salivary TAC was significantly lower in periodontitis and OLP patients compared to the controls, representing a significant oxidative process occurring in the oral cavity (20). In the present study, salivary TAC levels were lower in the PTC patients, while the difference was not considered statistically significant. Our findings were indicative of a significant correlation between salivary and serum TAC levels in the PTC patients and control subjects (P < 0.004 and P < 0.003, respectively).
As mentioned earlier, MDA is an end product of lipid peroxidation. Oxidative damage to cells and tissues could be manifested through the serum level of MDA as the lipid peroxidation marker (8). In the current research, serum MDA level was significantly higher in the PTC patients. Similarly, the results obtained by Sheeba et al. demonstrated higher MDA concentrations in breast cancer patients compared to controls (21).
Moreover, Sadani et al. and Mano et al. observed an elevated level of MDA in PTC compared to normal thyroid tissues (13-22). In this regard, the findings of Kosova et al. indicated significantly higher MDA levels in pre- and post-thyroidectomy compared to controls, while the MDA level in post-thyroidectomy was significantly lower compared to pre-thyroidectomy, and the healthy subjects still showed significantly higher levels of MDA. Higher levels of MDA could be attributed to the increased production of free radicals, exceeding the capacities of the antioxidant enzymes (23).
Although the PTC patients had higher salivary MDA levels in the present study, no statistically significant difference was observed between the case and control groups in this regard. The results obtained by Totan et al. showed a significant increase in the salivary and serum MDA levels in the OLP group compared to the controls (18). In addition, Onder et al. claimed that salivary MDA level was significantly higher in the patients with chronic periodontitis compared to the controls (24). In the current research, a correlation analysis was carried out to evaluate the salivary and serum levels of MDA in the PTC patients and control subjects. At the significance level of 0.7 for both specimens, the findings indicated that saliva samples could not be a proper substitute for blood samples in the monitoring of the MDA status.
Lack of significant differences between the salivary and serum MDA and TAC levels in the case and control groups in the present study could be due to the ultrafiltration on plasma. Ultrafiltration is a mechanism that involves the seeping-through of the plasma molecules via the spaces between the acinus and ductal cells and tight junctions between the secretory unit cells. The components entering the saliva through ultrafiltration are also affected by the dilution of the saliva flow rate as compared to those of the plasma (25,26).
4.1. Conclusions
According to the results, serum MDA and TAC levels were significantly higher and lower in the PTC patients compared to the control subjects, respectively, which confirmed the increased oxidative stress and lipid peroxidation in the patients. The oxidant/antioxidant imbalance was considered to be the possible cause of the increased thyroid cancer risk. Therefore, MDA and TAC assessments could determine the severity of thyroid cancer. On the other hand, lack of a significant difference between the measured indexes of the analytes in the saliva in the PTC patients and control subjects could be attributed to ultrafiltration, indicating that saliva cannot be a proper substitute for serum or plasma in the investigations in this regard.
4.2. Limitations of the Study
Due to the financial difficulties, the number of the recruited volunteers in the study was limited. It is recommended that similar studies be conducted on larger sample sizes in order to obtain more accurate results.