1. Background
Breast cancer (BC) is the most commonly occurring form of cancer and the 5th leading cause of cancer-related death in women (1). The breast-conserving surgery (BSC) dissection coupled with radiation therapy (RT) is accepted as standard care for the selected BC management (2, 3). Intraoperative radiation therapy (IORT) is a novel approach to BC treatment (4). Seroma contains several growths and inflammatory factors due to physiologic responses to the operation and wound healing process (5). Some studies suggest that seroma induces an increase in cell proliferation, migration, and invasion in the BC cell line (6-8). However, the proteomics analysis of IORT-treated seroma has shown inhibition of cell proliferation and invasion in the BC cell line (9, 10). To the best of our knowledge, there is no data available on the efficacy of seroma dependent on dose-time and source of IORT.
2. Objectives
In this study, we compared the cellular and molecular effects of IORT-treated seroma at the point of IOeRT versus IOxRT on the BC cell line.
3. Methods
3.1. Patients and Sampling
After taking informed consent the seroma samples were collected from 3 groups of patients according to clinicopathological data (Appendix 1 in Supplementary File) and the American Society for Radiation Oncology (ASTRO) criteria (11). Seroma samples were collected from 3 groups of IORT-treated patients, each group consisted of 3 patients. The first group received IOeRT (Boost dose=12Gy), the second group received IOeRT (Radical dose=21Gy) and the last group received IOxRT (X-ray=20Gy) in a cancer research center hospital in Tehran, Iran. The seroma from each group was collected in tubes containing protease inhibitors before being centrifuged (300g for 5min), sterile filtered (0.22 and 0.45 µm), pooled, and stored at -80°C for further analysis.
The Ethic committee of Shahid Beheshti University approved this study (code: IR-SBMU.RETECH.REC.1397.561).
3.2. Proliferation Assay
Immortalized human BC cell lines: MCF-7(ER/PR+; Her2/Neu), MDA-MB-231 (ER/PR-; Her2/Neu-) as well as MCF10 (as a control non-tumorigenic epithelial cell line) were obtained from Biological Research Center.
Cell viability was assessed after the cells were treated with different concentrations of seroma in an FBS-free medium for 24, 48, and 72 h. The concentrations were selected based on previous studies (12). The 96-well culture plates were used to seed the cells with a density of 1 × 104 cells/well. Two different concentrations of seroma (5 and 10 %) were used to treat the cells. The colorimetric development was quantified spectrophotometrically at 570 nm with an ELX800 microplate reader (BioTek Instruments Inc). The percentage of cell viability after 24, 48, and 72 h of IORT-treated seroma were calculated by the mean absorbance of viable treated cells divided by the mean absorbance of viable control cells × 100.
3.3. Apoptosis Assay
An Annexin V FITC Apoptosis Detection Kit I (eBioscience™ Annexin V Apoptosis Detection Kit FITC, USA) was used to investigate how MDA-MB-231 cells are influenced by the apoptotic effects of seroma. The seroma with a concentration of 10% from each group was used for the treatment of MDA-MB-231 cells for 2 days. The cells were centrifuged (2000 rpm × 5 minutes) and 100 μL of binding buffer was used to wash the pellets after removing the supernatant. Afterward, the cells were incubated for 15 minutes on ice in a dark condition and mixed with 5 μL of PI and 5 μL of Annexin V. After loading 400 μL of the binding buffer, a BD FACSCalibur™ flow cytometer (Becton Dickinson, USA) was used for analysis. The untreated cells were regarded as the negative control.
3.4. Cell Cycle Assay
Flow cytometry was used to investigate how cell cycle distribution is influenced by seroma compounds. Generally, 10% of seroma in the DMEM cell culture medium was used for the treatment of 4 × 105 cells for 2 days. Using centrifugation (2000 rpm, 5 min), the cells were collected. Thereafter, to the cells to stable the pellets, 500 μL of cold ethanol was added. Afterward, 1 mL of PBS-EDTA-BSA was added to the cells and centrifuged. After the addition of 2 mg/mL EDTA to PBS and performing autoclave, the PBS-EDTA-BSA solution was prepared, which was, then, used (10 mL) to liquefy BSA (0.1%) and refine the BSA solution into PBS-EDTA pellets were immediately washed, using the washing buffer prepared by mixing PBS (100 mL), BSA (1 g), EDTA (20 mg) and sodium azide (100 mg). Next, the staining buffer prepared by mixing PBS (1 mL), PI (0.3 μg/mL), RNase (50 μg/mL), Triton X-100 (1 μL/mL) and EDTA (0.37 mg/mL) was added. Incubation was done on ice for 30 min, and the cells were analyzed, using a flow cytometer. Flowjo software was run to analyze the percentage of cells in the G1, S, and G2 phases.
3.5. Migration and Invasion Assay
The MDA-MB-231 cells were treated with IORT-treated seroma, using individual and pooled samples. The Mitomycin as a scratch test and the Matrigel as a transwell test were used for studying the migration and invasion effects of IORT-treated seroma, respectively (13, 14).
3.6. Biomarker Test
For the investigation of the invasion and migration potential of MDA-MB-231 cells, we used a molecular test. For detection of cell senescence, P16 and were used; MMP-9 was used for detecting invasion as well. They were added, during the night at 4°C; following the rinsing at room temperature for 1 h, and inoculation was done with secondary antibodies. The end of reactions is reached following DAB staining and microscopic observation. The next step was hematoxylin re-dyeing. Hydrochloride alcohol was used for differentiation before implementing dehydration and xylene transparency in 4 y. Images were randomly taken from 3 fields per well; fluorescence microscopy did the counting.
3.7. Data Analysis
GraphPad Prism was used for analysis. The differences between the groups were analyzed, using Independent Student’s t-test (P < 0.05). All data are presented as the mean ± standard deviation (SD) of at least 2 independent experiments.
4. Results
4.1. Cell Proliferation
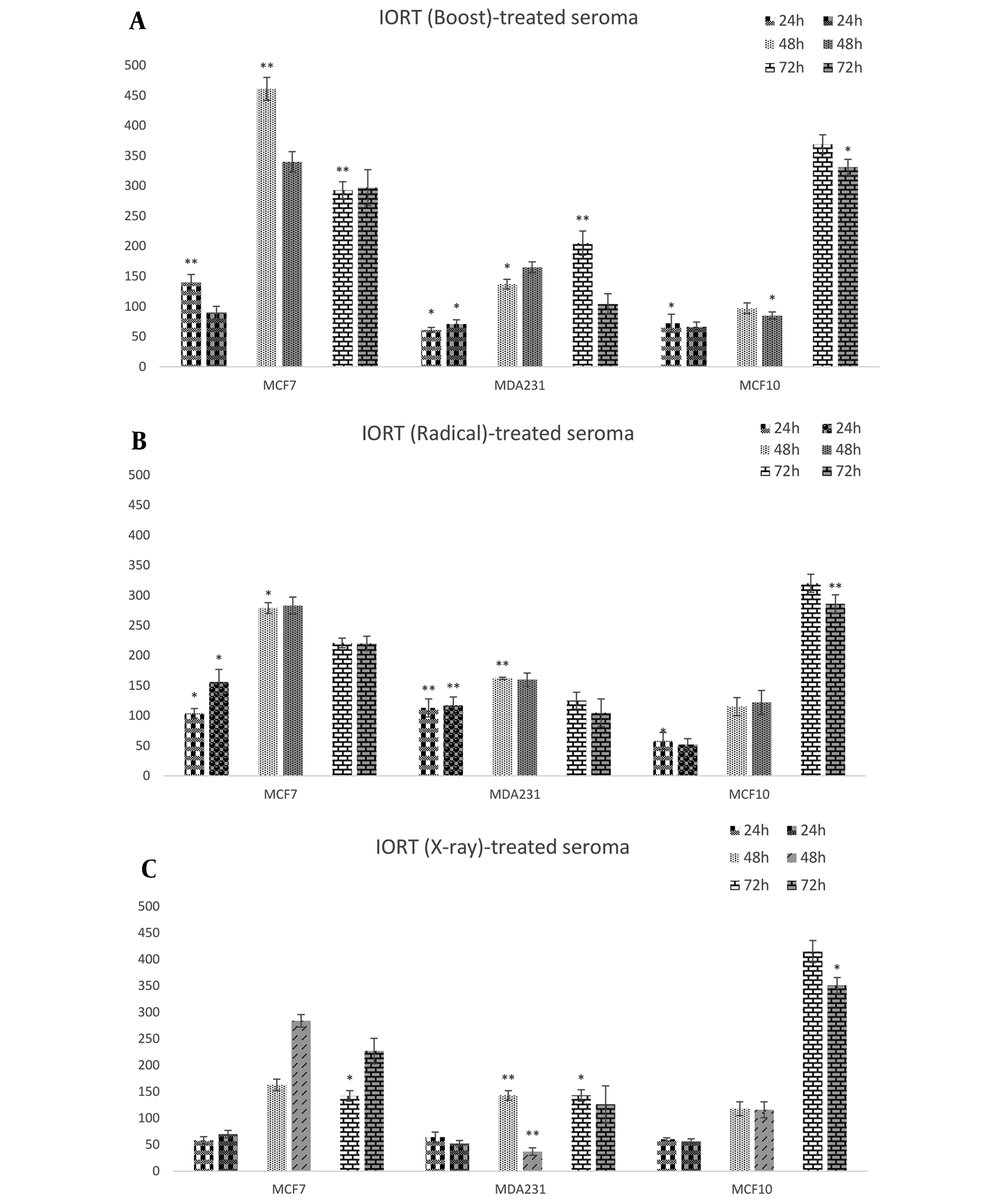
Three cell lines were incubated with seroma from 3 different IORT interventions (Boost, Radical, and X-ray) in a concentration-dependent manner. The MTT assay was used as a cell viability test. As indicated in Figure 1A, the relative cell viability in Boost-treated seroma was decreased in MDA-MB-231 cell lines after 24 h of treatment with both 5% and 10% concentrations of seroma. Neither 48 nor 72 h had a reduced effect on the MDA-MB-231 cell line. In contrast, it increases cell proliferation. However Boost-treated seroma did not affect the cell viability of MCF7.
As shown in Figure 1B, in Radical-treated seroma, no significant cell proliferation reduced effects on different BC cell types were observed. Figure 1C shows that X-Ray-treated seroma has a reduced effect on the proliferation of BC types of cell lines in 24 h. Furthermore, the MDA-MB-231 cell line shows a reduced effect on cell viability during 48 h.
4.2. Cell Cycle Arrest
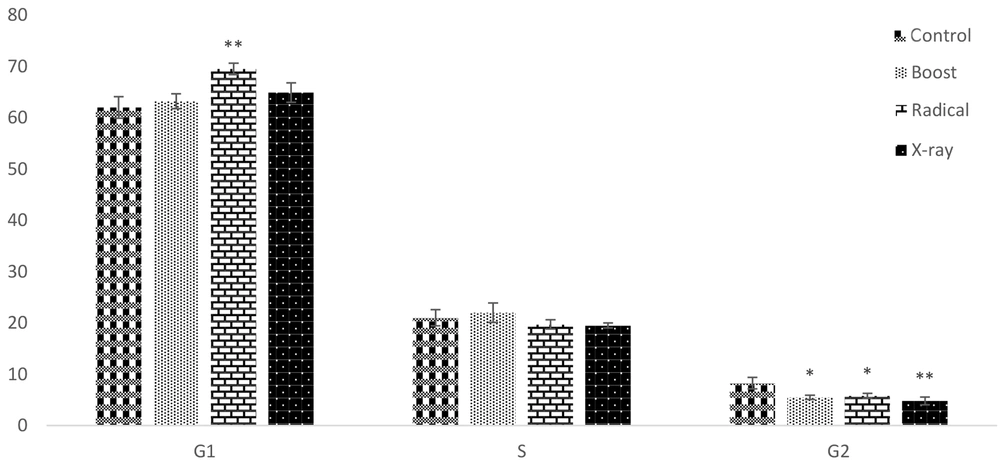
The PI staining followed by flow cytometry analysis was performed for the observed effect of IORT-treated seroma on the cell cycle arrest. For this purpose, the MDA-MB-231 cell line was used because of different tumorigenic aggressive phenotypes (15). As shown in Figure 2, no significant change occurred in the S phase. However, Boost, Radical, and X-Ray-treated seroma were arrested at the G2 phase compared to the control group. Additionally, the G1 phase was significantly arrested in the Radical group.
The PI staining followed by flow cytometry analysis was performed for the observation effect of IORT-treated seroma on the cell cycle arrest in MDA-MB-231 cells. No significant change occurred in the S phase. However, IORT (Boost, Radical and X-Ray)-treated seroma were arrested at G2 phase compared to the control group. Additionally, the G1 phase was significantly arrested in the Radical group.
4.3. Apoptosis Assay
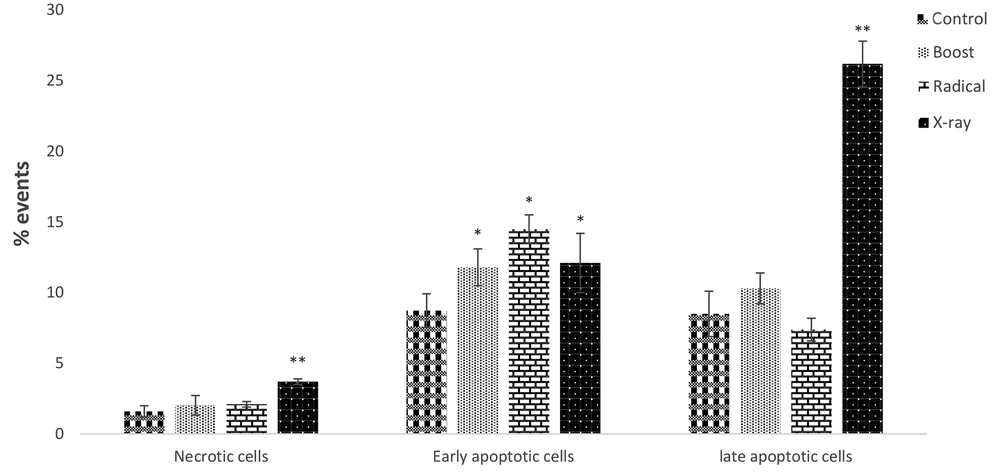
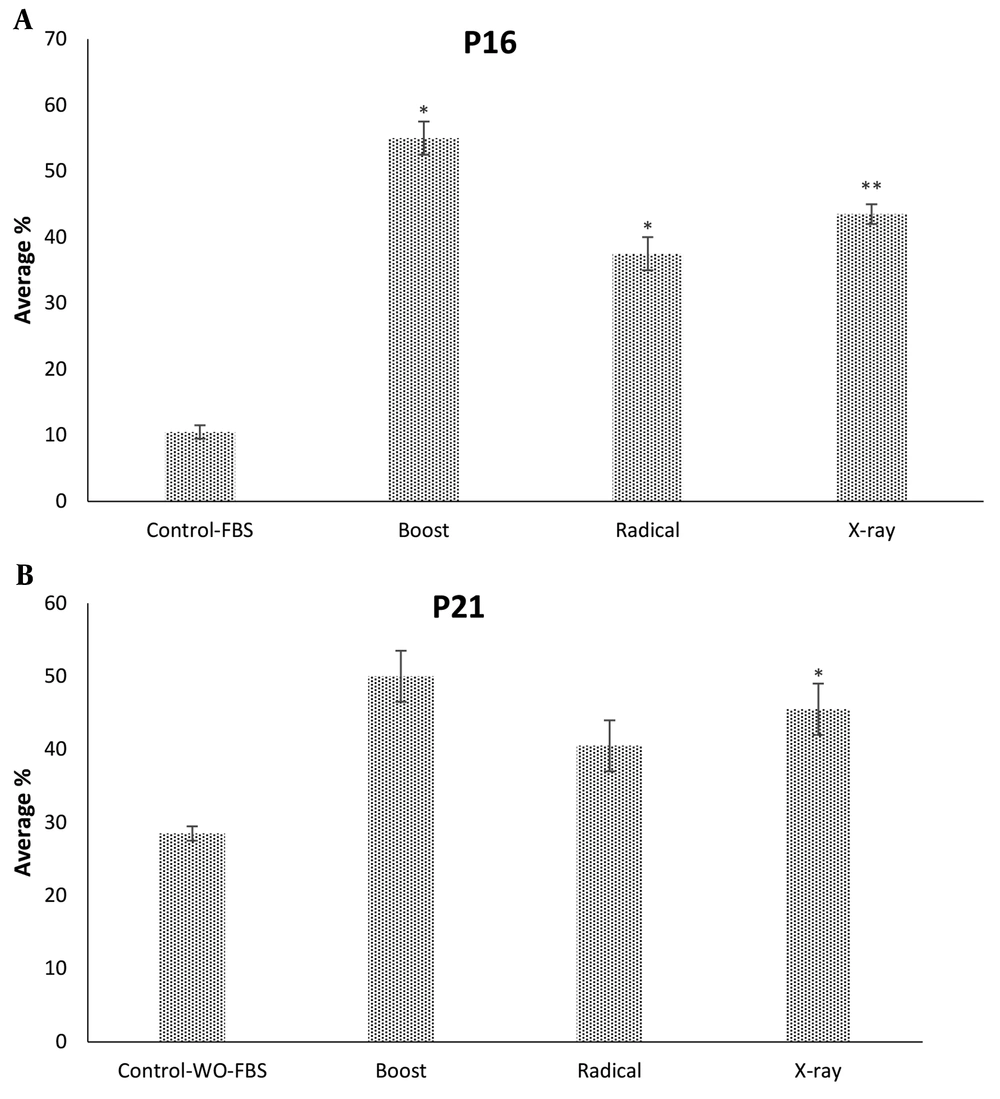
The apoptotic effects of IORT-treated seroma were investigated in the MDA-MB-231 cell line. As shown in Figure 3, compared to the control group, the Boost, Radical, and X-Ray-treated seroma increased early apoptotic. Furthermore, late apoptotic was increased significantly in the X-Ray-treated seroma group. Additionally, the expression level of P16 and P21 proteins (known as an apoptosis biomarkers) was analyzed. Figure 4A shows that Boost, Radical, and X-Ray-treated seroma upregulated the P16 protein expression level compared to the control group. Furthermore, Figure 4B shows that in the case of P21 protein expression level is significantly upregulated by X-Ray-treated seroma compared to the control group.
The apoptotic effects of IORT-treated seroma were investigated in the MDA-MB-231 cell line.The IORT (Boost, Radical and XRay)-treated seroma increased early apoptotic compared to the control group. Furthermore, late apoptotic was increased significantly in the IORT (X-Ray)-treated seroma group.
The expression level of P16 and P21 proteins we analyzed in MDA-MB-231 cells. A, IORT (Boost, Radical and X-Ray)-treated seroma upregulate the P16 protein expression level compared to the control group. B, P21 protein expression level is significantly upregulated by IORT (X-Ray)-treated seroma compared to the control group.
4.4. Migration Analysis
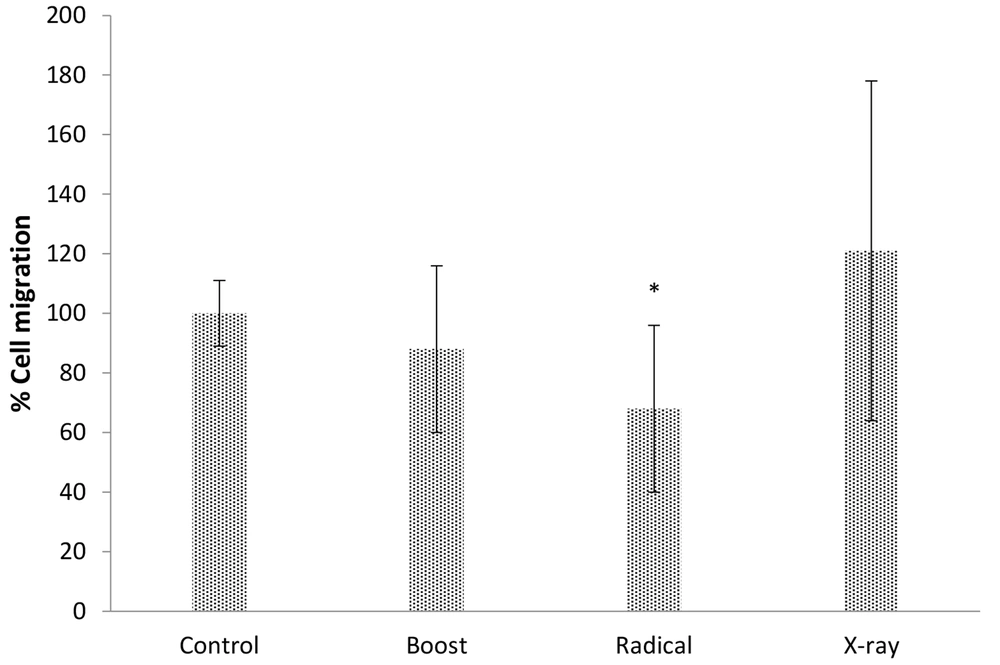
The scratch assay was used for studying the migration effect of IORT-treated seroma on the MDA-MB-231 cell line. Figure 5 shows the outcome of IORT-treated seroma on the MDA-MB-231 cell line. Accordingly, the Radical-treated seroma has the most significant effect on the inhibition of migration.
4.5. Invasion Effect
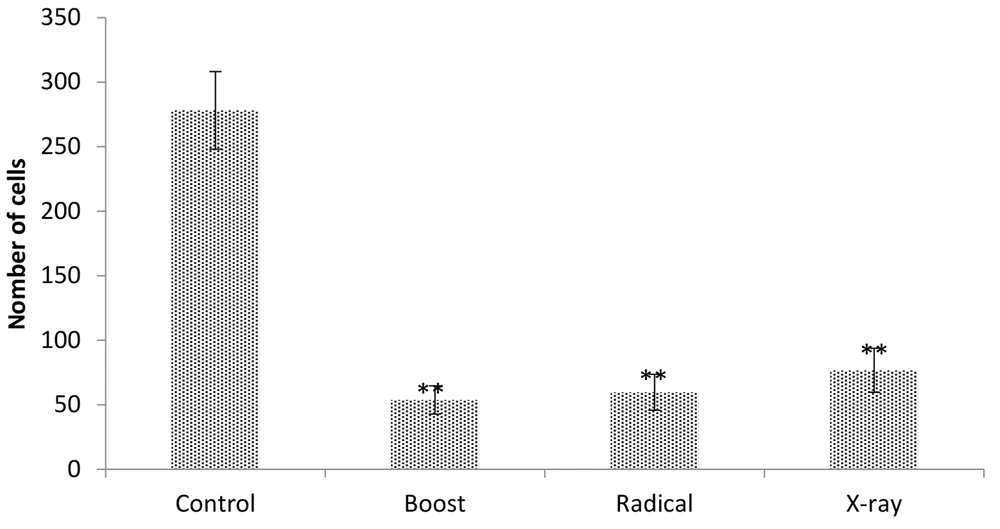
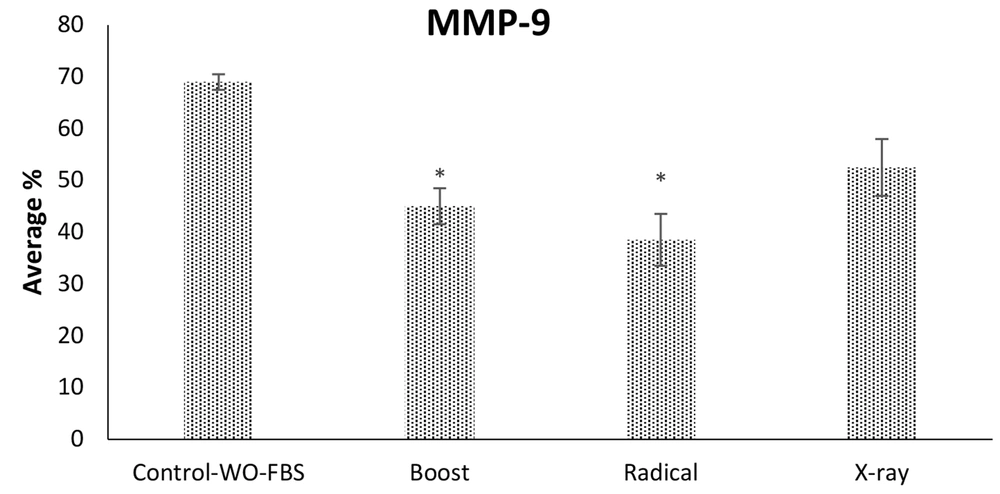
The MDA-MB-231 cell line was investigated for IORT-treated seroma effects on the invasion. Figure 6 shows that Boost, Radical, and X-Ray-treated seroma significantly reduced the invasion compared to the control group. Furthermore, the invasion effect was analyzed by expression level of MMP-9 protein. Figure 7 shows that the MMP-9 expression level was reduced in IOeRT-treated seroma but not in the IOxRT-treated seroma compared to the control group.
5. Discussion
The physiological responses to the BC operative trauma and the process of wound healing have contributed to the inflammatory effects. Additionally, these developments cause local recurrence (16, 17). Limited studies have shown that the treatment of tumor beds with IORT can produce seroma with different compositions compared to the non-IOR-treated seroma (15, 18). In the current study, we evaluated the effects of dose-time and source-dependent IORT-treated seroma on BC cell lines.
Studies have shown that the effectiveness of IORT-treated seroma can be dose-time and source-dependent IORT-treated seroma depends on the cancer cell line. The inhibition of cell proliferation, migration, colony-formation, and invasion are such effects (9, 15, 18).
According to our results, in the IOxRT-treated seroma group, it significantly reduces the proliferation rate of MCF7, MDA-MB231, and MCF10 after 24 h. Additionally, IOeRT (Boost)-treated seroma causes a significant decrease in the proliferation of MDA-MB231 and MCF10 and not that of MCF7. However, IORT (Boost, Radical, and X-ray)-treated seroma does not have a significant effect on the reduction of the proliferation rate for 48 h and 72 h. We suggest that IORT (Boost and X-ray)-treated seroma has protective effects in the tumor bed region during the first 24 h after exposure. However, this effect vanished over time.
Herskind et al. showed that IORT-treated seroma has less inhibitory effects on MCF-7 cells at 1% concentration compared to 3% (19). However, based on our observation, we have shown that IORT (Boost and X-ray)-treated seroma is independent of seroma concentration.
We have analyzed the cell cycle arrest by the exposure of MDA-MB-231 to IORT (Boost, Radical, and X-ray)-treated seroma. Bravata et al. have shown cellular senescence, which limits the proliferation of cell lines exposed to IORT. Our results show that IORT (Boost, Radical, and X-Ray)-treated seroma causes a cell cycle arrest, more significantly in the IOxRT-treated seroma G2 phase. Moreover, Bravata et al. have shown no apoptosis in the IORT-treated seroma cell line (18). However, we have observed that apoptosis occurs in the IORT-treated seroma cell line in the early apoptotic phase more significantly than in the IOxRT-treated seroma late apoptosis phase. Additionally, P16 and P21 proteins, which are apoptotic and cell proliferation arrest biomarkers expression levels, were measured (20). We observed an upregulation of these biomarkers in all groups more significantly in the IOxRT-treated seroma.
It has been shown that seroma can cause the migration and invasion of the cancer cell lines (6-8). Our results indicate that IORT-treated seroma causes an inhibition in the migration and invasion of the cancer cell lines. We have analyzed the expression level of MMP-9, which is an invasion biomarker. It is suggested that MMP-9 is upregulated in metastasis (21). Our results show that MMP-9 is downregulated in IORT (Boost, Radical, and X-Ray)-treated seroma.
We hypothesize that by the modification of the tumor bed, IORT causes changes in the proteome or metabolome profile of seroma. In this regard, it has been reported in previous studies that 21-gene recurrence score (RS) were evaluated for prognostic and predictive benefit in IORT patients (22); in addition, a value study indicated that key molecular pathways in radiotherapy (RT) are equally enriched by both Boost and Radical doses (23).
In summary, IORT (Boost, Radical, and X-Ray)-treated seroma has a significant effect on the proliferation, cycle arrest, death, migration, and invasion of the cell. Additionally, IOxRT-treated seroma has the most biological effects compared to IOeRT-treated seroma. However, this type of investigation would need to be extended to numerous patients; thus, the present work should be considered a pilot study.
5.1. Conclusions
There are two types of IORT, one delivers electron beams (IOeRT) and the second is known as low-kv-ray (IOxRT). To the best of our knowledge, there is no comparison of these two types of IORT based on cellular and molecular evidence. In this study, we observed that IORT (specifically IOxRT)-treated seroma has the most significant effects on the reduction of proliferation, induced cell cycle arrest, and apoptosis.
We have shown that IORT-treated seroma reduces proliferation after 24 h. However, we did not observe a reduction of proliferation at 48 h and 72 h. We suggest that at 24 h, the IORT-treated seroma may play an important protective role in the margin, which is followed by local recurrence decreases. However, this type of investigation would need to be extended to numerous patients; thus, the present work should be considered a pilot study. Also, we hypothesize that by modification of tumor bed, IORT causes changes in the proteome or metabolome profile of seroma. Therefore, based on our study, we propose the drain be clamped up to 24 h after irradiation. Although this is a pilot study, we suggest investigating the importance of IORT-treated seroma, more patients, and research with long-term follow-up needed.
In addition, suggesting the importance of IOxRT-treated seroma was not only inferior compared to IOeRT-treated seroma, but also was more biological effective in the BC cell line.