1. Background
Type 2 diabetes mellitus (T2DM) is the most common chronic metabolic disease in postmenopausal women (1). Accumulating evidence from clinical studies shows that menopausal-diabetic women have increased hospital admission rates due to cardiovascular diseases (CVDs) (2, 3). Metabolic disorders (such as increased free fatty acid (FFA) oxidation) play a major role in diabetes-induced CVD (4, 5). Cardiac metabolism constantly shifts between glucose and FFAs depending on the metabolic states, and this cardiometabolic flexibility is important for regular cardiovascular function and development (6). Loss of cardiac metabolic flexibility in favor of more rigid substrate utilization and toward increased FA metabolism has deleterious metabolic consequences and may be detrimental to cardiac function (7). It has been shown that in menopausal insulin-resistant rats, an increase in sterol regulatory element-binding protein-1 (SREBP-1) increases CD36 as a fatty acid transporter and decreases the peroxisome proliferator-activated receptors (PPARs) as a molecular target for metabolic disease, thereby worsening the metabolic profile and increasing the size and content of adipose tissue (8). The reason for diabetic cardiomyopathy is the changes in metabolic and hormonal factors, such as abnormal consumption of FFAs by cardiomyocytes, which leads to a disproportionate response to conditions such as ischemia. Both a decrease and an increase in FFA intake make the heart more susceptible to lipotoxic dysfunction (4, 9).
Clinical findings show that premenopausal women are more resistant to CVD than men (1) and premenopausal-diabetic women (3) of the same age. However, the incidence of CVD increases after menopause (3). It is now known that the increase in the incidence of CVD after menopause is associated with the loss of protective function of sex hormones, especially estrogen. In addition, menopause has been shown to be associated with decreased insulin secretion and clearance, which alters insulin levels in the bloodstream. Accordingly, it appears that endogenous estrogen protects against CVD in premenopausal women (10).
The protective effects of estrogen on metabolism and cardiovascular function are mediated through a variety of pathways and molecules that prevent visceral obesity (11), inflammation, hyperinsulinemia, insulin resistance (12), and hypertension (13). 17β-Estradiol (E2), as the main female sex hormone, has 3 receptors: estrogen receptor α (ERα), estrogen receptor β (ERβ), and G protein-coupled estrogen receptor (GPER) (14). ERα and ERβ act through the genomic pathway, while GPER acts through the non-genomic pathway and is responsible for the rapid effects of E2 (15). Studies have shown that GPER has cardiovascular protective (16) and anti-diabetic effects (17) in a postmenopausal diabetic condition, but its mechanism of action is not well understood. Based on genetic and pharmacological approaches, a growing body of experimental evidence highlights the role of GPER in E2 function and, in particular, the adjustment of metabolism and cardiovascular function (17).
Today, we know that diabetic cardiomyopathy is the leading cause of death in postmenopausal diabetic patients. With the discovery of new mechanisms of this disease (mainly related to impaired cardiac metabolism), new treatments for this disease are needed. Also, the results of our previous study (15) showed that GPER has cardiovascular protective effects in a postmenopausal diabetic condition.
2. Objectives
In the present study, we aimed to determine whether the observed protective effects of this receptor in a postmenopausal diabetic condition are due to changes in lipid metabolism in the heart.
3. Methods
3.1. Experimental Design
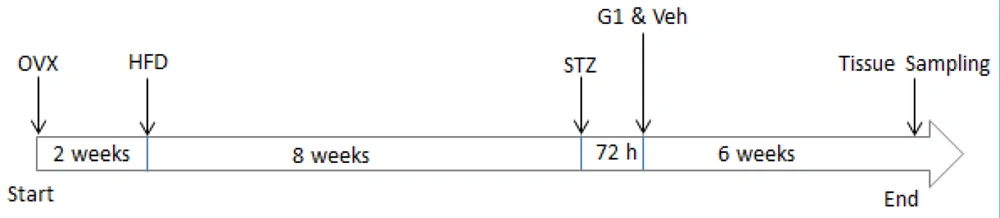
Our current research was performed on 35 female Wistar rats (3 months old with a weight of 200 - 250 g). The animals were housed in a 12: 12-hour light-dark cycle at 22 - 23°C in accordance with the National Institute of Health guidelines. All experimental protocol was approved by the Animal Care and Ethics Committee for Experimental Animals at Shahid Sadoughi University of Medical Sciences, Yazd, Iran (code: 1400.125). The female animals were divided into 5 groups (n = 7 in each group): Sham-control (Sh-Ctl), T2DM, ovariectomized-T2DM (OVX-T2DM), OVX-T2DM-G1 (GPER-agonist), and OVX-T2DM-vehicle (OVX-T2DM-Veh). Experimental menopause was induced by bilateral ovariectomy. Following anesthesia through intraperitoneal injection of ketamine/xylazine (80/10 mg/kg), a 2- to 3-cm-long incision was made in the abdomen. Skin and muscles were opened, and the uterus was removed. Finally, 1 mL of saline was poured into the abdomen, and the muscles and skin were sutured. Removing the ovaries simulates the menopause state in rats, and the success of ovariectomy was confirmed by radioimmunoassay determination (Hangzhou, Eastbiopharm, China) of serum levels of E2. The inter-assay coefficient of variation was less than 10%. To ensure that the effects of E2 were eliminated, all animals were ovariectomized 2 weeks before the experiment (18). Type 2 diabetes mellitus was induced by a combination of a high-fat diet (HFD) and streptozotocin (STZ), as described previously (19). In summary, female rats were placed on a modified HFD for 8 weeks. Then, rats were injected with a dose of STZ (30 mg/kg; Sigma USA). After 72 hours, blood samples were collected from their tails, and rats with a fasting blood sugar (FBS) of ≥ 300 mg/dL were considered diabetic and included in the study. After confirmation of T2DM, a vehicle (0.1% dimethyl sulfoxide [DMSO]; Sigma USA) and G1 (200 μg/Kg; Tocris Bioscience, USA) were administered intraperitoneally 3 days per week for 6 weeks (16). Body weight was recorded at the end of the experiment. The schematic representation of the experimental protocol is illustrated in Figure 1.
3.2. Measurement of Plasma Insulin
At the end of the experiment, blood was sampled from the cardiac ventricle while the rats were under deep anesthesia. Then, the blood sample was centrifuged, and the plasma was separated to measure insulin levels. The level of plasma insulin was measured by a rat ELISA kit (Hangzhou, Eastbiopharm, China) with a sensitivity of 0.05 mIU/L and an inter-assay coefficient of variation of less than 10%.
3.3. Determination of Free Fatty Acid Concentrations
At the end of the therapy period, animals were anesthetized with ketamine/xylazine (80/10 mg/kg), and their hearts were removed and weighed. Then, left ventricles (100 mg) were homogenized in phosphate-buffered saline (PBS), and protein concentration in the homogenates was measured by the Bradford assay. Cardiac FFA contents were measured by a Cu-colorimetric method using a non-esterified fatty acid assay kit (ZellBio GmbH, Ulm, ZB-FFA-48A, Germany) with a sensitivity of 5 μmol/L, 95% confidence interval (CI) level, and an inter-assay coefficient of variation of 6%.
3.4. RNA Isolation and Real-time Polymerase Chain Reaction
Total RNAs from the left ventricles were extracted with acid phenol-guanidinium thiocyanate chloroform extraction (TRI Reagent R, Sigma, USA). Electrophoresis on 2% agarose gel was used to verify the quality of RNA. Complementary DNA (cDNA) was synthesized using a reverse transcription kit (TaKaRa, Japan). Quantitative real-time polymerase chain reaction (PCR) was utilized by the SYBR Master Mix (Thermo Fisher Scientific, Germany) on the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Germany). Messenger RNA (mRNA) expression levels were calculated using formula 2-ΔΔCt (where ΔΔCt = ΔCt sample-ΔCt reference). The primers used in this section are listed in Table 1.
| Genes | Primer Sequences | Accession Number | Annealing (°C) | Size (bp) | Cycle |
|---|---|---|---|---|---|
| PPARα | F: 5’-GGGACAAGGCCTCAGGATACCACTA-3’; R: 5’- GACATCCCGACGGACAGGCACT-3’ | XM_039078501.1 | 64 | 184 | 45 |
| CD36 | F: 5’- AGGAAGTGGCAAAGAATAGCAG-3’; R: 5’- ACAGACAGTGAAGGCTCAAAGA-3’ | XM_039108092.1 | 58 | 163 | 45 |
| GAPDH | F: 5’- AACGACCCCTTCATTGAC-3’; R: 5’- TCCACGACATACTCAGCAC-3’ | XM_039107008.1 | 58 | 191 | 45 |
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
3.5. Oil Red O Staining
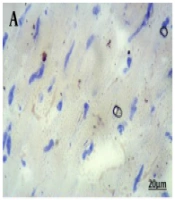
Oil red O staining was used to quantify the lipid contents in cardiac tissue. Cardiac sections (4 μm) were fixed with 2% formalin (5 minutes) and then rinsed with BPS. The cardiac sections were then treated with 20% isopropanol (2 minutes) and, after that, incubated with 0.3% (w/v) oil red O solution (Sigma Aldrich, USA) in 60% isopropanol (20 minutes). Cellular nuclei were stained with Mayer’s hemalum solution (2 minutes). A high-resolution image was taken using a microscope (Olympus, Tokyo, Japan). Lipid contents were quantified as lipid area (positive area of oil red O) as a percentage of total tissue area.
3.6. Statistical Analysis
The data were reported as mean ± SEM. For statistical analysis, the data were performed using 1-way analysis of variance (ANOVA), followed by the Tukey-Kramer test. P values less than 0.05 were considered statistically significant.
4. Results
Table 2 shows changes in body weight and glycemic profile in different groups. Type 2 diabetes mellitus increased body weight and plasma insulin (P < 0.01) and FBS levels (P < 0.001) compared to Sh-Ctl animals. The removal of ovaries exacerbated the effect of T2DM on body weight, resulting in a significant difference between the OVX-T2DM group and the T2DM group (P < 0.05). Treatment with GPER-agonist G1 prevented body weight gain in OVX-T2DM animals, resulting in a significant difference between the OVX-T2DM-G1 group and the OVX-T2DM-Veh group (P < 0.05).
Abbreviations: BW, body weight; E2, 17β-estradiol; FBS, fasting blood sugar; G1, GPER-agonist; Ins, insulin; OVX, ovariectomized; Sh-Ctl, sham-control; T2DM, type 2 diabetes mellitus; Veh, vehicle.
a Data are expressed as mean ± SEM (n = 7 per group).
b P < 0.01
c P < 0.05 vs T2DM
d P < 0.05 vs OVX-T2DM-Veh
e P < 0.001 vs Sh-Ctl
f P < 0.001
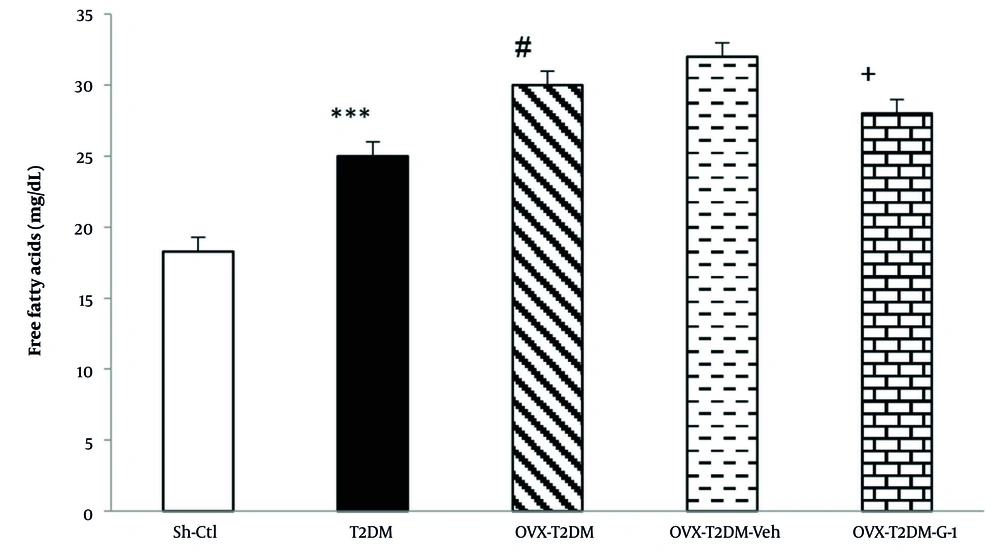
The effects of G1 treatment on the cardiac contents of FFAs are presented in Figure 2. Type 2 diabetes mellitus markedly raised the cardiac levels of FFAs compared to the Sh-Ctl group (P < 0.001). Figure 2 shows that ovariectomy in T2DM animals caused a further increase in the content of cardiac FFAs (P < 0.05). In contrast, administration of G1 significantly reduced the levels of cardiac FFAs in ovariectomized T2DM rats (P < 0.05).
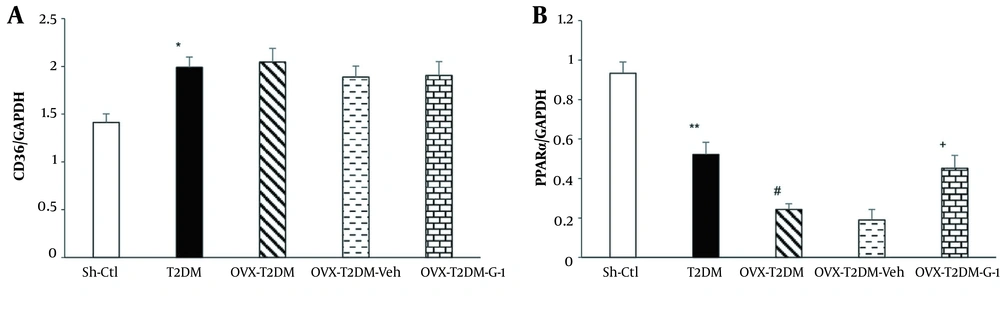
Figure 3A shows the changes in CD36 mRNA expression in different study groups. As can be seen, induction of T2DM by HFD-STZ increased the mRNA expression of CD36 compared to the Sh-Ctl group (P < 0.05), while neither ovariectomy nor G1 administration had any impact on it.
A, relative cardiac mRNA expressions of CD36 and; B, peroxisome proliferator-activated receptor α. Mean ± SEM (n = 7 per group). *P < 0.05 and **P < 0.01 vs Sh-Ctl, #P < 0.05 vs T2DM, +P < 0.05 vs Veh. Abbreviations: G1, GPER-agonist; OVX, ovariectomized; Sh-Ctl, sham-control; T2DM, type 2 diabetes mellitus; Veh, vehicle.
In another part of the research, changes in the cardiac peroxisome proliferator-activated receptor α (PPARα) mRNA expression were measured. As shown in Figure 3B, induction of T2DM in female animals reduced PPARα mRNA expression compared to the Sh-Ctl group (P < 0.01). Also, when both diabetes and ovariectomy were present, a further decrease in PPARα mRNA levels was observed (P < 0.05). In contrast, treatment with GPER-agonist G1 (OVX-T2DM-G1) counteracted the effects of ovariectomy and T2DM and increased PPARα mRNA expression compared to the OVX-T2DM-Veh group (P < 0.05).
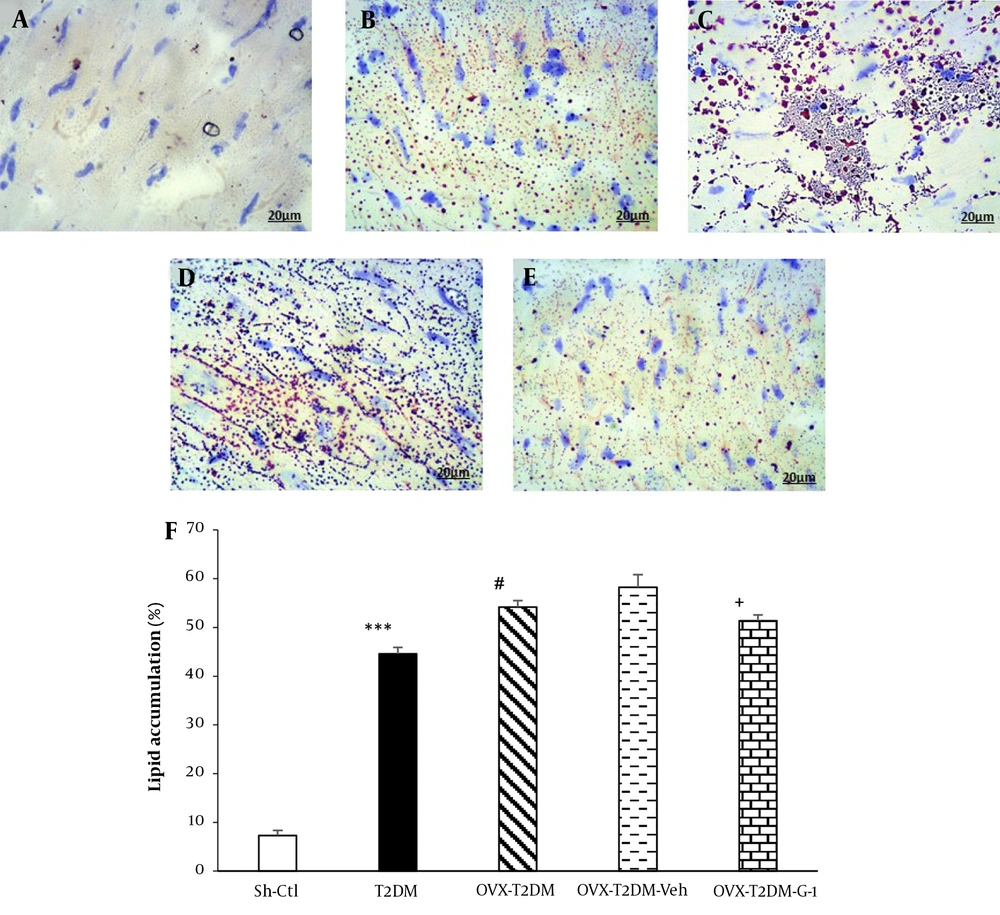
In the last section of the current research, changes in the myocardial lipid accumulation were investigated and quantified using ImageJ software. As shown in Figure 4F, chronic T2DM increased cardiac lipid content compared to the Sh-Ctl group (P < 0.001). The concurrent presence of T2DM and menopause led to a further rise in myocardial lipid content compared to the T2DM rats (P < 0.05), while in the G1-therapy group, the myocardial lipid content was lower than the OVX-T2DM-Veh group (P < 0.05).
Intracellular lipid accumulation in the left cardiac ventricle. The microscopic images of oil-red-O-stained myocardial left ventricle tissue were obtained from female rats A, Sh-Ctl; B, T2DM; C, OVX-T2DM; D, OVX-T2DM-Veh; and E, OVX-T2DM-G1. F, the pictures were analyzed by ImageJ software. Red spots designate the place of lipids. Scale bar: 20 μm. Data are shown as mean ± SEM (n = 5). ***P < 0.001 vs Sh-Ctl; #P < 0.05 vs T2DM; +P < 0.05 vs OVX+T2DM+Veh. Abbreviations: G1, GPER-agonist; OVX, ovariectomized; Sh-Ctl, sham-control; T2DM, type 2 diabetes mellitus; Veh, Vehicle.
5. Discussion
E2 and, in particular, GPER has been widely considered the treatment of choice for metabolic and cardiac disorders in postmenopausal diabetic women (17). The protective cardio-metabolic effects of GPER in diabetic patients (17) and menopausal-diabetic rats (15) have recently been reported, but the mechanisms involved are not yet well understood. Since both diabetes and menopause are associated with changes in cardiac metabolism, which lead to functional changes in the heart and reduced cardiac function (16, 20), in this study, we used a model of postmenopausal diabetes to determine the impact of selective GPER agonist on cardiac lipid metabolism as a possible protective mediator. The most important findings of this study are as follows: First, T2DM increased the accumulation of lipids within cardiomyocytes by increasing the cardiac level of FFAs and the expression of CD36, as well as decreasing the expression of PPARα. Second, induction of the menopausal model (ovariectomy) exacerbated the effects of diabetes on cardiac FFAs, CD36, and PPARα, which ultimately resulted in the accumulation of more lipids within cardiomyocytes. Third, chronic stimulation of GPER reduced lipid accumulation within cardiomyocytes by reducing cardiac FFAs and increasing PPARα.
Our results showed that induction of diabetes increased body weight, and ovariectomy aggravated these changes. Also, treatment with GPER-agonist G1 reduced body weight. It is established that diabetes causes hyperphagia (21), and ovariectomy causes weight gain in diabetic rats (22, 23), as they eliminate the anorexigenic effects of estrogen (11). Furthermore, G1 reduces body weight in ovariectomized animals with obesity and diabetes (17). This result is consistent with the findings that show GPER deficiency results in reduced energy expenditure and weight gain in female mice (24). Also, G1 may exert a centrally mediated thermogenic effect by activating the sympathetic nervous system, which causes weight loss (17).
In our and other models of diabetes T2DM, insulin and glucose levels will rise, mimicking human T2DM (25, 26). In this study, similar to other studies that have used the HFD-STZ model of T2DM (27), plasma insulin levels increased, indicating insulin resistance. Similar to this study, other studies have shown that induction of menopause and stimulation of GPER in OVX-diabetic animals has no effect on insulin secretion (15).
One of the causes of diabetic cardiomyopathy is changes that occur in the metabolic status of the heart, which is accompanied by a lack of energy, low glucose concentration, and increased FFAs (28). Free fatty acids are less efficient fuels and increase insulin resistance by inhibiting insulin signaling (6), and this insulin resistance and reduced glucose uptake by cardiomyocytes increase FFAs and accumulation of excess metabolites (20). In agreement with the results of our study, other studies have shown that ovariectomy is associated with increased cardiac (29) and circulatory (30) FFAs. Also, Sharma et al. (31) showed that GPER-deficient rats had higher levels of FFA profiles, which was associated with higher insulin resistance. They suggested that GPER-selective agonists could be used in this way to improve CVD caused by diabetes and obesity (31).
Free fatty acids are able to pass through the membrane and enter the cells by facilitated diffusion, which this process is saturated and inhibited by proteases. In addition, there are 3 types of FFA transporters in the heart, including CD36, FATP, and FABP, of which CD36 is the major transporter (32). As in this study, it was confirmed that increased delivery and availability of FFAs in diabetic conditions increased the expression of FFA transporters (such as CD36) in cardiomyocytes, which in turn increases FFA uptake (32). Increased expression of CD36 and FABP is present in both diabetic animals and humans, which increases the oxidation capacity of FFAs and triglyceride storage by facilitating the absorption of FFAs (33, 34). Also, in STZ-induced type 1 diabetes, CD36, and FABP proteins increase (32). The data concerning GPER-agonist effects on cardiac CD36 are exceptionally rare. Similarly, it has been shown that stimulation of ERs could not alter CD36 mRNA expression (35). Given that in the present study, only CD36 mRNA changes were examined, it is possible that the effect of E2 and ERs-agonists on the CD36 gene resulted in changes in the CD36 protein level in cardiac cells. It is well documented that E2 and ERs-agonists activate signaling pathways that regulate protein metabolism (35). Some reports contradict the results of this study. For instance, Martinez-Cignoni et al. (36) showed that induction of ovariectomy in diabetic animals was associated with decreased CD36 expression. The reason for these contradictory results can be due to the difference in the type of injury and duration of diabetes.
PPARs are a group of ligand-activated transcription factors that alter the consumption of FFAs at the transcriptional level when activated. Peroxisome proliferator-activated receptor α is the primary regulator of FFA metabolism in the heart, and in general, activation of cardiac PPARα increases the oxidation and consumption of FFAs (32). Similarly, it has been shown that in chronic hyperglycemia, glucose decreases PPARα, accompanied by increased lipid esterification and the inability of myocytes to use FFAs, resulting in the accumulation of intracellular lipids. This accumulation leads to an increase in non-oxidative productions (such as ceramide), which is a toxic lipid compound (28). Contrary to our findings, it has been reported that diabetes increases cardiac PPARα (9). The reason for these contradictory results can be the difference in the duration of diabetes, as well as the animal’s sex and age.
Similar to the present study, it was reported that obese and insulin-resistant ovariectomized animals had lower levels of the PPARα protein and mRNA (37, 38). Chen et al. (39) showed that the loss of ovarian estrogen by downregulation of PPARα reduced energy production and weakened the myocardial structure. Peroxisome proliferator-activated receptor α is a ligand-activated transcription factor that belongs to the family of steroid hormone receptors; since obese female animals have higher levels of PPARα than obese male animals (40), it appears to have effects on diabetes, obesity, and lipid metabolism with sexual dimorphism, and possibly estrogen is a contributing factor (41). Recent findings suggest that there may be a signal between PPARα and estrogen receptors responsible for the effects of E2 on diabetes, obesity, and lipid metabolism (41). Since PPARα increases in diabetic hearts, it plays a compensatory role in maintaining ATP production (9). Estrogen and its receptor agonists are likely to affect the metabolic status of postmenopausal diabetic hearts. In addition, estrogen is able to regulate lipid metabolism by increasing the expression of enzyme genes, such as pyruvate dehydrogenase kinase 4 (PDK4) and carnitine palmitoyl transferase I (CPT1) (42). In general, PPARα and GPER are important partners in the estrogenic state, and both act via the PI3K-Akt pathway (43).
To our knowledge, despite the growth of preclinical evidence, there is still no adequate clinical support for the use of GPER-agonist as part of the therapeutic arsenal for the treatment of such diseases.
The current study had some limitations that should be addressed. The first limitation of this study was the use of rats to model human type 2 diabetes because conducting long-term, well-controlled interventional studies is far more complicated than using animal models. Second, the consequences and efficacy (for example, potential toxicity) of long-term G1 treatment, as would be required in humans to ameliorate diabetes, are currently unknown and require further preclinical and clinical studies. The last limitation of this study was the lack of measurement of protein expression.
5.1. Conclusions
In summary, this is the first study to investigate the effects of GPER agonists on cardiac lipid metabolism in OVX-T2DM female rats. Our results showed that in a postmenopausal diabetic condition, G1 as a selective GPER agonist has a beneficial cardiometabolic role. This, in part, could be related to a decrease in the levels of FFAs and lipids and an increase in the expression of PPARα in the heart, improving the cardiometabolic status of the postmenopausal diabetic model. We conclude that G1 is a prototype candidate drug for potential translation into clinical applications. Future translational studies in women will ultimately confirm the effects of G1 on postmenopausal diabetic patients.