1. Introduction
Subcutaneous insulin resistance syndrome (SIRS), initially described by Schneider and Bennett, is defined as a syndrome in which a patient poorly responds to subcutaneous (SC) insulin but maintains a normal response to intravenous (IV) insulin (1). The pathophysiological underpinnings of this syndrome are yet to be fully delineated, with increased SC insulin-degrading activity (2, 3) or impaired insulin transportation/absorption (4) being the most prominent suggested mechanisms. Treatment options for SIRS include intramuscular (IM) insulin, inhaled insulin, intraperitoneal insulin infusion via an insulin pump, continuous SC infusion of insulin lispro plus heparin, continuous SC infusion of ultra-rapid insulin lispro, and β-cell transplantation (4-9). Here, we report two cases of SC and IM insulin resistance adequately managed with a combination of dapagliflozin and IM insulin. To our knowledge, these are the first reported cases in which a sodium-glucose cotransporter-2 (SGLT-2) inhibitor was used to manage SIRS.
2. Case Presentation
2.1. Case 1
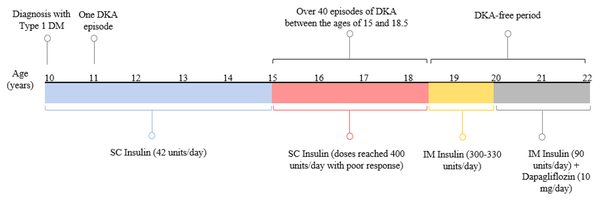
A 22-year-old Caucasian female patient (body mass index: 20.9 kg/m2) diagnosed with type 1 diabetes at the age of 10 years was initially well-managed with 12 units of SC insulin glargine per day and ten units of regular insulin before each meal three times a day. Apart from one episode of diabetic ketoacidosis (DKA) one year after diagnosis, she was healthy and achieved good glycemic control with an HbA1c of 6.5 - 7.5% (48 - 58 mmol/mol) over 5 years following her diagnosis. At age 15, the patient started to experience recurrent episodes of DKA successfully managed with IV insulin. Between the ages of 15 and 18.5 years, the subject was admitted to different hospitals with more than 40 episodes of DKA (Figure 1).
Although the patient would quickly respond to IV insulin, her blood glucose levels would consistently re-escalate as she was transitioned back to SC insulin, necessitating dramatic increases in SC insulin doses. Insulin non-compliance was ruled out by proper dosing and administration by a healthcare professional. In addition, there was no clinical evidence of lipodystrophy upon clinical examination, and anti-insulin and anti-insulin receptor antibody assays yielded negative results. Different types of insulin were administered subcutaneously, including rapid-, short-, intermediate- and long-acting insulins in various regimens, with total doses reaching 400 unit/day. However, the patient did not achieve glycemic control, as evidenced by her fasting and postprandial plasma glucose levels, which ranged from 300 to 500 mg/dL. According to these observations, the case was diagnosed clinically with SIRS.
The patient was treated with regular insulin and insulin glargine administered intramuscularly in different areas. The response was poor despite administering a total of 400 units of insulin daily. Finally, IM injections of 10 units of regular insulin were administered hourly, in addition to 120 units of IM insulin glargine per day. Insulin injections were not frequent during her sleeping hours, with a total insulin dose of 300 - 330 unit/day. This regimen was successful in terms of glycemic control, with fasting and postprandial plasma glucose levels of 100 - 120 mg/dL and 140 - 180 mg/dL, respectively. The HbA1c of the patient dropped from 10 - 11% (86 - 97 mmol/mol) to 7 - 8% (53 - 64 mmol/mol) over the following few months and was stabilized at these values thereafter. She was DKA-free throughout this regimen.
At the age of 20, 10 mg/day of dapagliflozin was added as an adjunct therapy to IM insulin (Figure 1) in an attempt to reduce insulin requirements with less frequent painful IM injections. Strikingly, adding dapagliflozin significantly reduced IM insulin dose from 300-330 unit/day to 90 unit/day (70% dose reduction) administered as 10 unit/6 h of regular insulin and 50 unit/ day of insulin glargine. Since adding dapagliflozin, the patient has been DKA-free (Figure 1) with only one urinary tract infection. Table 1 summarizes plasma glucose levels for case 1 on different insulin regimens.
| Regimen 1 | Regimen 2 | Regimen 3 | Regimen 4 | |
|---|---|---|---|---|
| Total daily dosage | 400 unit/day of SC insulin | 400 unit/day of IM insulin | 300-330 unit/day of IM insulin a | 90 unit/day of IM insulin+10 mg/day of dapagliflozin |
| Plasma glucose levels (mg/dL) | 300 - 500 | 300 - 400 | FBG: 100 - 120; PPBG: 140 - 180 | FBG: 100 - 140; PPBG: 140 - 200 |
Plasma Glucose Levels for Case 1 on Different Insulin Therapy Regimens Following Her Diagnosis with Subcutaneous Insulin Resistance Syndrome
2.2. Case 2
A 21-year-old female patient diagnosed with type 1 diabetes at 18 years presented with a clinical profile similar to the first case. She was diagnosed with SC insulin resistance at the age of 20. Her HbA1c was 12% on 350 - 400 unit/day of SC insulin, and her case was complicated by recurrent episodes of DKA. The patient required a total IM insulin dose (regular and glargine insulins) of 350 - 400 unit/day yet also with poor glycemic control.
Similar to the first case, the addition of 10 mg/day of dapagliflozin significantly decreased her IM insulin dose from 350 - 400 unit/day to 120 unit/day (66% dose reduction) administered as 15 units/6 h of regular insulin and 60 unit/day of insulin glargine. Since adding dapagliflozin, the patient has been DKA-free with adequate glycemic control and an HbA1c of 7 - 8%.
3. Discussion
Our patients were diagnosed clinically with SIRS. They demonstrated IM insulin resistance as well. This is not unusual because augmented insulin-degrading activity in muscle has been reported in SIRS (3), suggesting that SIRS may also have coexisting elements of IM resistance as a part of the same syndrome. Dapagliflozin is an SGLT-2 inhibitor that inhibits glucose reabsorption in the proximal kidney tubule, producing glucosuria. Adding 10 mg/day of dapagliflozin to our patients’ regimens resulted in a significant decline in the total daily IM insulin dose by at least 70% and 66% for the first and second cases, respectively. The latter percentages exceed those of other clinical trials whose insulin dose reduction reached 22% with 10 mg/day of dapagliflozin (10), indicating that dapagliflozin may have effects other than glucosuria.
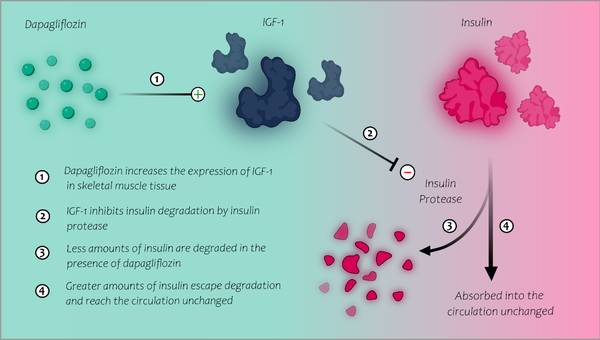
An insulin protease has been identified in human muscle and SC tissue (11) and is believed to be responsible for insulin destruction in these tissues in patients with SIRS. Misbin et al. (12) demonstrated that IGF-2 and, to a lesser degree, IGF-1 inhibit the insulin-degrading activity of this insulin protease. Their study reported a case of SIRS whose serum demonstrated increased insulin-degrading activity presumed to be a consequence of "leakage" or "spillover" of insulin-degrading enzymes from the patient's SC tissue. Incubation of their patient's serum with normal serum inhibited the insulin-degrading activity, suggesting that SIRS patients may be deficient in a circulating protease inhibitor in normal patient sera. The serum analysis of the SIRS patient showed low levels of IGF-1 and IGF-2. Therefore, experimental restoration of normal IGF-2 levels in the patient’s serum abolished the insulin-degrading activity. Moreover, the addition of IGF-1 to the patient’s serum resulted in similar inhibition to a lesser extent. As a result, low levels of IGF-1 and IGF-2 seemed to be responsible for the increased insulin-degrading activity in the muscle and SC tissue of that patient. Interestingly, dapagliflozin has been shown to restore pulsatile growth hormone secretion in obese mice and increase IGF-1 mRNA expression in their muscle tissue (13). In the light of these findings, we hypothesize that dapagliflozin, by possibly raising IGF-1 mRNA expression in skeletal muscle, may augment the inhibitory effect on insulin degradation in skeletal muscle, explaining the substantial IM insulin dose reduction in our patients (Figure 2).
3.1. Conclusions
These are the first reported cases of SIRS to be successfully treated with an SGLT-2 inhibitor and IM insulin therapy. The main limitation of this study was the lack of analysis of IGF-1 and IGF-2 serum levels in our patients to support our hypothesis further. However, it is unknown whether serum IGF-1 and IGF-2 correlate with their levels in skeletal muscle in patients with SIRS. Nonetheless, dapagliflozin appears to have impacts other than glucosuria when administered with IM insulin, as observed in both patients. Additional studies are required to support further the efficacy of dapagliflozin and possibly other SGLT-2 inhibitors in managing SIRS.