1. Background
Diabetes is one of the most prevalent endocrine diseases worldwide, and the number of people with diabetes has more than doubled over the past 30 years (1). Diabetes is characterized by beta cell dysfunction and absolute or relative insulin deficiency due to both genetic and environmental causes (2-6), however, the exact pathophysiological mechanism is still not known. Genome wide association studies have described around 250 gene regions predisposing to type 2 diabetes (7), of which more than 90% are genes related to beta cell function (8, 9). Knowledge of beta cell function is therefore of great importance in understanding diabetes pathophysiology. Insulin secretion is tightly regulated by glucose, however, other nutrients and hormones also affect insulin secretion, including vitamin D (10). Vitamin D is primarily known for its vital role in calcium homeostasis, however, many extra-skeletal effects of vitamin D have become apparent the last years, including effects on beta cells (11). Vitamin D is synthesized endogenously in the skin by UV exposure or derived from foods and supplements. The major form of vitamin D in the circulation is 25(OH) vitamin D, which is metabolized primarily in the kidneys by 1-alpha-hydroxylase to generate the active form of vitamin D, 1,25(OH)2 vitamin D. 1-alpha-hydroxylase is also expressed in several extra-renal tissues in humans including skin, gastrointestinal tract, placenta, and in the pancreatic beta cells (12, 13). This enables local production of 1,25(OH)2 vitamin D and permits intracellular regulation of vitamin D levels, also in beta cells (12, 14). 1,25(OH)2 vitamin D performs the majority of its effects through regulation of gene expression in different tissues and cells through binding to the nuclear vitamin D receptor (VDR), which acts as a transcription factor (15).
A number of studies have indicated a beneficial role for vitamin D in pancreatic beta cell function. In vivo studies have shown reduced secretion of insulin from pancreatic islets in mice suffering from vitamin D deficiency. The insulin secretion was shown to improve by vitamin D supplementation (16). Evidence has also been presented indicating that vitamin D can protect beta cells from cytokine induced apoptosis (16, 17). Microarray studies of mice islets have identified genes regulated by 1,25(OH)2 vitamin D, among them genes involved in ion transport, lipid metabolism, and insulin secretion (17). Previous studies have reported divergent results on the effect of insulin secretion after treatment with 1,25(OH)2 vitamin D. Wolden-Kirk et al., did not show an increase in insulin secretion after preincubation of mouse islets with vitamin D followed by stimulation with high levels of glucose (18), whereas Jeddi et al., reported that preincubation of rat islets with vitamin D combined with a high glucose concentration increased glucose stimulated insulin secretion (19). However, the effects of vitamin D combined with glucose stimulation on insulin secretion in beta cells have not been fully elucidated, especially regarding the difference of effect between vitamin D metabolites. We have recently performed a study to determine the effect of 25(OH) vitamin D and 1,25(OH)2 vitamin D on the proteome of the INS1 cell line. The study showed that 31 proteins were differentially expressed after treatment with 1,25(OH)2 vitamin D, whereas 25(OH) vitamin D had no such effect (20). Among the upregulated proteins were proteins implicated in insulin granule motility and insulin exocytosis, suggesting a positive effect of 1,25(OH)2 vitamin D on insulin secretion (20) .
2. Objectives
The aim of the present study was to assess whether vitamin D would influence glucose stimulated insulin secretion in INS1E beta cells and whether there is a difference in the effect of 1,25(OH)2 vitamin D and 25(OH) vitamin D on insulin secretion.
3. Methods
3.1. Culturing INS1E Cells
We used the well-established rat insulinoma cell line INS1E and treated the cells with 25(OH) vitamin D and 1,25(OH)2 vitamin D prior to glucose stimulation. INS1E is a transformed cell line from rat insulinoma often used as a model for studies of beta cell function and presents an improved cell line compared to INS1. Studies have shown stable growth of INS1E cells over two years in culture and physiological insulin response to glucose, in contrast to INS1 cells (21).
The cell-line was a kind gift from professor Wollheim (21). INS1E cells were grown adherently in RPMI medium (Gibco by Life Technologies, Paisley, UK) with supplements (FBS 10%, Na-pyruvate 0.01 M, HEPES 0.1 µM, penicillin/streptomycin 10 mL and 2-mercaptoetanol 300 µL per 30 mL RPMI) in a humidified atmosphere at 37°C with 5% CO2. RPMI was changed two to three times per week and cells sub-cultured to 80% confluence. INS1E cells were grown for four weeks until stable log phase growth. Cells (105 per well in 12-well plates) were seeded in RPMI with supplements. We performed three parallel studies with triplicates.
3.2. Stimulation with Glucose After Pre-Treatment with Vitamin D Metabolites
After four passages, INS1E cells were incubated with RPMI with 10 nM 25(OH) vitamin D (Sigma-Aldrich, Netherlands), RPMI with 10 nM 1,25(OH)2 vitamin D (Sigma-Aldrich, Israel), or RPMI alone (control cells). The applied concentrations of 25(OH) vitamin D and 1,25(OH)2 vitamin D were chosen based on results from previous studies (18, 22). Vitamin D was dissolved in ethanol before the given amount was added to the medium. The RPMI, with supplements and vitamin D was replenished once during the 72 hours of preincubation. After 72 hours of preincubation, glucose stimulation was carried out. After gentle removal of RPMI, the cells were incubated with glucose-free Krebs-Ringer buffer for 60 minutes. Cells were subjected to stimulation with (1) Krebs-Ringer buffer or Krebs-Ringer buffer supplemented with (2) 5 mM glucose, (3) 11 mM glucose, or (4) 22 mM glucose in triplicates for 60 minutes. After stimulation, the medium was collected immediately to stop the incubation and centrifuged at 2000 g for 10 minutes. The supernatant was kept at -18°C prior to insulin measurement. The cells were collected by gentle mechanical release, resuspended until homogenous, and counted in an automated cell-counter (Countess, BioRad). The experiment was performed three times over a period of four weeks. The average cell numbers for each experiment are shown in Table 1.
| Control Cells | 1,25(OH)2 Vitamin D + 22 mM Glucose | 25(OH) Vitamin D + 22 mM Glucose | |
|---|---|---|---|
| Experiment 1 | 7.5 × 105 | 5.5 × 105 | 4.5 × 105 |
| Experiment 2 | 5.8 × 105 | 6.0 × 105 | 4.2 × 105 |
| Experiment 3 | 6.1 × 105 | 6.8 × 105 | 6.6 × 105 |
Average Cell Numbers for Experiments 1 - 3
3.3. Insulin Assay RIA Kit
Insulin was measured by a RIA kit (Millipore, Missouri, U.S.A.), according to manufacturer’s protocol.
3.4. Statistics
None of the parallels were excluded in the calculations. The cells not stimulated with vitamin D metabolites were considered as reference cells in each group. All data were expressed as mean ± SD and analysed using IBM SPSS 23.0. To calculate P values, a two-tailed student t test was applied. P values below 0.05 were regarded as statistically significant.
4. Results
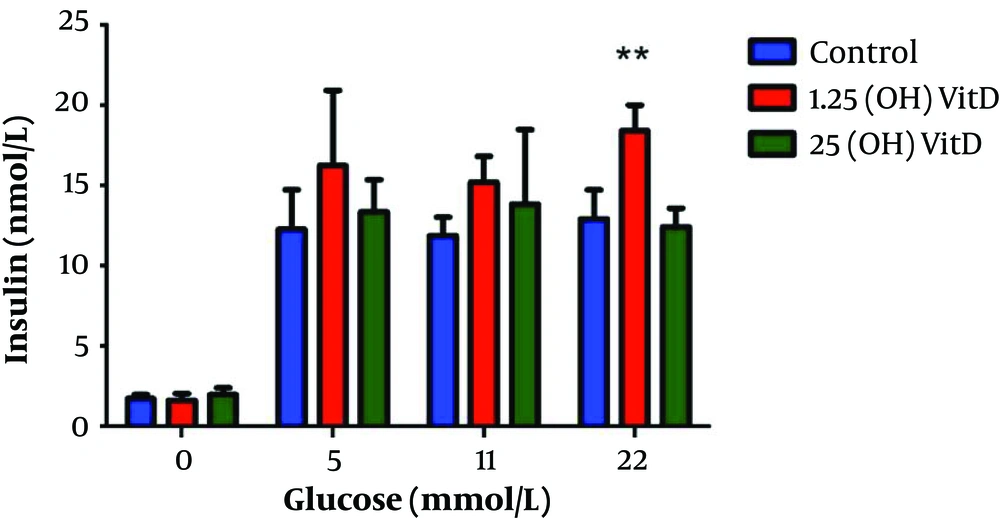
As shown in Figure 1, there is an increased insulin secretion with increasing levels of glucose in the control cells, in cells preincubated with 1,25(OH)2 vitamin D, and in the cells preincubated with 25(OH) vitamin D. The cells treated with 1,25(OH)2 vitamin D and stimulated with 22 mM glucose showed a 43% increase in insulin secretion compared to control cells (P = 0.006). Stimulation with 22 mM glucose after preincubation with 25(OH) vitamin D on the contrary showed no effect on insulin secretion. Neither treatment with 1,25(OH)2 vitamin D nor 25(OH) vitamin D lead to changes in insulin secretion in the absence of glucose stimulation.
Insulin release in response to treatment with different vitamin D metabolites at different glucose concentrations. Insulin release in response to one hour glucose stimulation at 0, 5, 11, and 22 mM, respectively, after preincubation with vehicle, 25(OH) vitamin D, or 1,25(OH)2 vitamin D for 72 hours. ** P < 0.05 compared to control (no vitamin D).
The INS1E cells treated with 1,25(OH)2 vitamin D showed a trend towards increased insulin secretion at all glucose concentrations. At 5 mM glucose, there was a 32% increase in the amount of insulin secreted compared to control cells. At 11 mM glucose, a 28% increase was observed. No significant changes were detected when comparing control cells with those pre-treated with 25(OH) vitamin D for any glucose concentrations.
The average cell number for each experiment is shown in Table 1. At 22 mM glucose, the average change in cell numbers between the control cells and the cells treated with 1,25(OH)2 vitamin D was -0.36 × 105 cells (-2.0 - 0.7 × 105 cells). The average change in cell number comparing control cells and cells treated with 25(OH) vitamin D was -1.37 × 105 (- 3.0 - 0.5 × 105 cells). Thus, both preincubation with 25(OH) vitamin D and 1,25(OH)2 vitamin D led to a slight decrease of cells.
5. Discussion
This study has shown that treatment of INS1E cells with 1,25(OH)2 vitamin D prior to glucose stimulation at high doses (22 mM) leads to significantly increased insulin secretion compared with control cells. In contrast, preincubation with 25(OH) vitamin D did not alter the glucose stimulated insulin secretion significantly, regardless of the glucose concentration. We found no changes in insulin secretion between the cells preincubated with vitamin D metabolites or vehicle in the absence of glucose. To our knowledge, studies on the effect on insulin secretion of different vitamin D metabolites in INS1E cells have not been carried out before. Yet, these results are in line with several other studies showing an association between 1,25(OH)2 vitamin D and insulin release from beta cells (19, 22-25). Billaudel et al. (23), reported that glucose stimulated insulin secretion was reduced in islets from vitamin D deficient rats, whereas incubation with 1,25(OH)2 vitamin D had a stimulatory effect on insulin response after six hours. Tanaka et al. (24), showed that insulin secretion was decreased in vitamin D deficient rats, but restored to the level of the controls in rats treated with vitamin D. Bourlon et al. (25), also reported that insulin secretion was diminished in islets from vitamin D deficient rats and restored by 1,25(OH)2 vitamin D combined with 16.7 mM glucose stimulation. Consistent with our results, d’Emden et al., showed a 2.5 fold increase of insulin secretion in response to 10 nM 1,25(OH)2 vitamin D in rat islets; this treatment had to last for 96 hours for the effect to become evident (22).
In 2015, Jeddi et al., reported that preincubation of rat islets for 24 or 48 hours with 1,25(OH)2 vitamin D increased glucose stimulated insulin secretion at high levels of glucose (16.7 mM), however, no effects on insulin secretion at low glucose levels were found (19).
Contrary to our results, Wolden-Kirk et al., reported no significant changes in insulin secretion after treatment with 1,25(OH)2 vitamin D on mice islets (18). In this study, preincubation with active vitamin D lasted for 24 hours and the glucose levels were either 3 mM or 30 mM. It might be possible that the difference in length of preincubation with vitamin D, the chosen glucose concentrations, and the use of mice islets in this experiment led to the observed differences between their study and ours.
The mechanisms of the effects of vitamin D on glucose stimulated insulin secretion in beta cells are not completely understood and both direct and indirect mechanisms have been suggested. Bourlon et al., demonstrated in their study that 1,25(OH)2 vitamin D could activate the de novo biosynthesis of insulin in rat islets and suggested this could be caused by increased rate of conversion of proinsulin to insulin (25). They also showed that vitamin D required 48 hours in culture to increase insulin secretion from beta cells, which could indicate a genomic effect of vitamin D in beta cells (25). Another study from Bourlon et al. (26), showed that 1,25(OH)2 vitamin D might have a modulatory role on insulin release from beta cells via the cyclic AMP pathway in rat, suggesting also non-genomic effects of vitamin D on insulin release. Vitamin D may stimulate a second messenger system that includes phospholipase C and G-protein receptors, which leads to increased calcium influx and increased intracellular glucose within the beta cells, causing insulin secretion (27). In addition, simulation of vitamin D receptor by 1,25(OH)2 vitamin D might diminish the dedifferentiation in beta cells seen in type 2 diabetes (10).
In the present study, treatment with 25(OH) vitamin D, in contrast to 1,25(OH)2 vitamin D, did not alter the insulin secretion significantly. This may suggest that the conversion of 25(OH) vitamin D into 1,25(OH)2 vitamin D is insufficient in INS1E cells. Our recent proteomic study carried out on INS1 cells to clarify the difference between 25(OH) vitamin D and 1,25(OH)2 vitamin D showed that only treatment with 1,25(OH)2 vitamin D significantly changed the expression of numerous proteins, including proteins that may affect insulin secretion. On the contrary no effect was seen on the protein expression for 25(OH) vitamin D (20). This is in line with our present study and supports the hypothesis that there will be a difference in the effects of different vitamin D metabolites on insulin secretion. The 1-alpha-hydroxylase protein was not detected in the study by Pepaj et al., indicating that this enzyme may not be expressed in INS1 cells, though additional studies are necessary to confirm these results (20).
INS1E cells are widely used as a model for pancreatic beta cells due to their stability in culture and their well-preserved glucose-induced insulin secretion within the physiological range (21, 28). However, it must be taken into consideration that the origin is a transformed cell line from the rat and will differ from islets regarding both function and mechanisms. INS1E cells lack the surrounding alpha and delta cells that normally reside in the pancreas. These are very important for the function of the beta cells and thus, for the secretion of insulin (29). Another limitation of the study is that the cells were preincubated with only 10 nM vitamin D metabolites. Future studies could possibly elucidate whether prolonged treatment of 1,25(OH)2 vitamin D, at even lower concentrations, would also increase glucose stimulated insulin secretion.
Vitamin D could potentially influence the number of INS1E cells in culture by increasing mitosis (30) or the apoptotic rate (31) and thereby, modify insulin secretion solely due to cell numbers. In order to avoid this bias, the cells were counted before and after treatment with vitamin D metabolites. The cell numbers remained stable throughout the study. A modest reduction of cell numbers was seen in the treated cells compared to the control cells.
In conclusion, the effect of preincubation of INS1E cells with vitamin D on insulin secretion appears to depend both on the vitamin D metabolite and glucose concentration. Only 1,25(OH)2 vitamin D increased insulin secretion, whereas 25(OH) vitamin D had no such effect. Future studies could clarify this observed difference and elucidate the possible mechanisms for the effect of 1,25(OH)2 vitamin D on glucose stimulated insulin secretion in beta cells.