1. Background
The potential effects of substance use on adolescent behavior and brain development are well documented by studies (1). Adolescence is a vulnerable time for starting a psychiatric disorder such as drug abuse (2). Drug abuse and depression are the most common disorders that often occur during adolescence (3).
Craving and negative emotions (such as stress, anxiety, and depression) are the main causes of continued substance consumption and returning to substances after withdrawal (4). Substance craving is one of the most important diagnostic criteria of substance use disorder (SUD) based on the fifth edition of the diagnostic and statistical manual of mental disorders (DSM-5) (5). Craving is one of the strongest and most stable causes, among other factors (6). For people with SUD, craving is the hardest challenge during withdrawal (7). Adolescents experience significant negative emotions in their lives (8). Studies show that substance abuse in adolescents is often associated with psychological distress, negative emotions, and psychiatric illnesses (9). Increasing comorbidity of negative emotions and SUD increases concerns (10) because depression and anxiety may not only affect the behavior for seeking help and joining the treatment (11) but also leads to a decrease in the quality of life (12), increases the risk of relapse (13), leads to social isolation (14), and ultimately increases the risk of death (15). Therefore, modulating drug craving and negative emotions (anxiety, stress, and depression) is seemed to prevent substance use in adolescents. The self-medication hypothesis introduced by Khantzian suggests that people use drugs and alcohol to regulate their emotions, such as anxiety, depression, stress, pain, loneliness, and so on (16).
Transcranial direct current stimulation (tDCS) is one of the main techniques for modulating brain activity and cortical excitability, which involves the use of a low-intensity electrical current over the scalp (17). Recently, some research has studied the implementation of tDCS for children and adolescents with various neuropsychiatric disorders. This new relevant literature addresses both safety and potential therapeutic efficiency in children and adolescents (18). Also, the interest has increased in non-invasive methods for stimulating brain activity in the treatment of addiction (19). Thus, tDCS has been used in numerous studies for improving negative emotions such as depression (20).
Today, mindfulness training is considered a promising treatment for various substance abuse disorders (21). Previous studies suggest that mindfulness-based interventions (MBIs) improve substance use disorders by increasing the cognitive regulation of several activity processes, including making clear cognitive appraisals and moderating negative emotions to decrease permanent cognition and emotional arousal, increasing cognitive to reduce drug-related attentional bias, reducing cue reactivity, and increasing cognitive control over craving (22).
Although tDCS can be effectively used as an alternative therapy, new studies have suggested it as a “strengthening therapy” to promote the effectiveness of other psychotherapy treatments (23). To investigate the efficacy of tDCS in combination with psychotherapy for the treatment of MD, Nejati et al. (24) proposed a new therapeutic approach called Psychological Intervention Combined with direct current electrical stimulation (PIN-CODES).
The review of the related studies shows that both mindfulness and tDCS are independently effective in treating addiction. Studies have also shown that the stimulation of the frontal area of the brain can enhance the effects of mindfulness by improving working memory (25). One hypothesis is that combining mindfulness therapy with electrical stimulation can have synergistic effects (26), but it has not yet been studied.
In the present study, we evaluated the effects of mindfulness (as a psychological intervention), tDCS, and the combination of tDCS and MBSAT (PIN-CODES) on negative emotions and reducing craving in adolescents with methamphetamine use disorders. Also, we expect tDCS + MBSAT to have longer-term effects compared to other treatments, especially tDCS (22-26).
2. Objectives
This is the first study of the effectiveness of the combination of psychological intervention and tDCS for the improvement of negative emotions and craving in adolescents with methamphetamine use disorders.
3. Patients and Methods
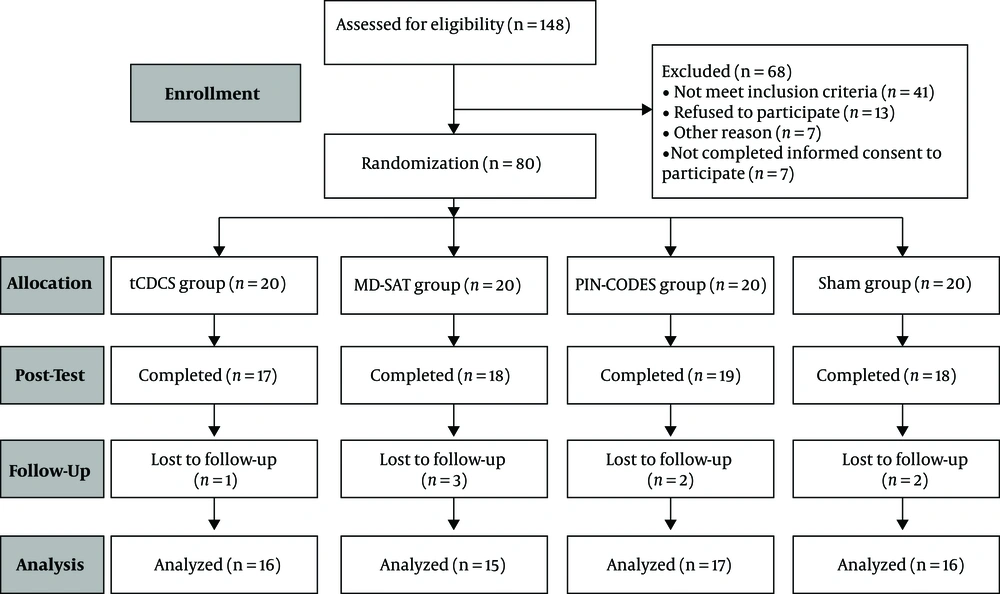
Eighty adolescent boys (Figure 1) aged 18 to 21 with early abstinent methamphetamine abuse were enrolled in the study (mean age = 19.46, SD = 1.15). Participants were randomly assigned to the research groups (tDCS group (n = 20), mindfulness group (n = 20), combined mindfulness-tDCS group (n = 20), and sham group (n=20)) by the block randomization method. The inclusion criteria were: (1) Referral to the addiction treatment center in Ardabil for quitting methamphetamine use; (2) methamphetamine use disorder diagnosis using clinical interviews by a psychiatrist based on DSM-IV criteria; (3) having 12 months of experience in methamphetamine abuse before referral to the addiction treatment center; (4) age range of 18 to 21 years; (5) avoiding any sedatives or stimulant drugs except for nicotine, as confirmed by a negative urine test; and (6) not taking antidepressants or other psychiatric drugs during the study. The exclusion criteria were: (1) Inability to tolerate tDCS; (2) comorbidity with axis I disorder such as bipolar disorder, schizophrenia, or any axis II disorders; (3) cranial or brain metal implant; and (4) history of seizures, epilepsy, brain injury, or any neurological disorders. The ethical principles in the present study were based on the latest edition of the Helsinki Declaration and approved by the Ethics Committee of Shahid Beheshti University (code: IR.SBU.ICBS.97/1036). Also, this study was registered in the Iranian Registry of Clinical Trials (no.: IRCT ID: IRCT20181013041327N1).
3.1. Procedure
The subjects in the electrical stimulation group received 12 sessions of electrical stimulation with a duration of 20 min, and the interval between the sessions was 72 h. The anode electrode was fixed over area F3 and area F4. The MBSAT group participated in the protocol of mindfulness consisting of 12 sessions (two sessions weekly). Each mindfulness treatment session lasted between 40 and 50 minutes. Finally, in the combination therapy group, participants received 12 sessions of electrical stimulation, followed by mindfulness therapy immediately after each electrical stimulation session. The sham group received 12 sessions of 20 minutes (0.0 mA; two sessions weekly) in six weeks with a 72-h interval between the sessions. The psychological intervention used in the present study consisted of Mindfulness-based Substance Abuse Treatment (MBSAT) (27). Table 1 shows the structure and content of each MBSAT session.
| MBSAT Session | |
|---|---|
| Session 1 | Introduction to the program: (1) informal greeting; (2) introduction to the program; (3) group agreements; (4) definition and description of mindfulness. (5) meditation: mindfulness of deep breathing; (6) group survey: teenagers’ learning interests; (7) homework and ending the session. |
| Session 2 | Mindfulness of health effects of drugs: (1) Central meditation; (2) mindfulness entry; (3) classification of drugs; (4) deadly drug compounds; (5) meditation: deep breathing; (6) homework and ending the session. |
| Session 3 | Reaction versus response: (1) Role-play: the power of the body versus the power of the mind; (2) discussion: reaction vs. response; (3) STIC (stop, take a deep breath, imagine the future consequences, choose) contemplation; (4) role-plays of STIC; (5) meditation: breath consciously; (6) mindfulness entry; (7) homework and ending the session. |
| Session 4 | Mindfulness of delusion: (1) Central meditation; (2) poem: “the perfect high; (3) enter consciously; (4) discussion: the positive and negative aspects of substance use; (5) negative and positive personal aspects of substance abuse; (6) meditation: body scan; (7) homework and ending the session. |
| Session 5 | Emotional awareness: (1) Central meditation: body scan; (2) classifying the types of emotions; (3) expressing emotions and their gender differences; (4) if you stand. (5) self-disclosure in depth; (6) game: concentration; (7) homework and ending the session. |
| Session 6 | The brain and drug abuse: (1) Central meditation led by adolescents; (2) enter consciously; (3) explaining the brain areas involved in addiction I; (4) meditation break; (5) explaining the brain areas involved in addiction II: substance use, trauma, and the mindful brain; (6) meditation: body scan; (7) homework and ending the session. |
| Session 7 | Mindfulness of craving: (1) Central meditation led by adolescents; (2) enter consciously; (3) practicing mindful eating; (4) the role and importance of craving in drug addiction; (5) practicing static body scan; (6) worksheet: causes and factors of craving; (7) homework and ending the session. |
| Session 8 | Mindfulness of triggers: (1) Central meditation led by adolescents; (2) enter consciously; (3) mindfulness of triggers; (4) three-level effects; (5) meditation: concentrate and pay attention to awareness; (6) homework and ending the session. |
| Session 9 | Family system and drug addiction: (1) Central meditation led by adolescents; (2) meditate my children; (3) negative effects of substance abuse on family relationships; (4) drug addiction and its intergenerational harm; (5) meditation: compassion and kindness to family members; (6) enter consciously; (7) homework and ending the session. |
| Session 10 | Mindfulness of pressure and the role of peers: (1) Playing the role of peer pressure; (2) discussion: friends vs. accomplices; (3) enter consciously with prompt; (4) mindful communication; (5) playing the role of peer pressure by adolescents; (6) meditation: practice compassion for friends and colleagues; (7) homework and ending the session. |
| Session 11 | Mindfulness of the understanding of external environment: (1) Central leadership by adolescents; (2) enter consciously; (3) mindfulness of the external environment; (4) transmitting systemic effects; (5) compassionate meditation towards the community; (6) homework and ending the session. |
| Session 12 | The end of the ceremony: (1) Meditation: final practice; (2) enter consciously. (3) group integration; (4) appreciate as a group; (5) party and celebration; (6) awarding a certificate; (7) completion of the training course |
3.2. Instruments
3.2.1. Depression, Anxiety, and Stress Scales (DASS-21)
These scales (DASS-21) were used to assess the negative emotions of adolescents with SUD (28). Evidence shows that the three scales of stress, anxiety, and depression have good convergent and discriminatory validity, as well as high internal consistency in both healthy individuals and those with clinical problems (alpha = 0.93 - 0.95) (28).
3.2.2. Desires for Drug Questionnaire
The Desire for Drug questionnaire (DDQ) was used to measure craving for drugs in persons with substance use disorders. Franken et al. (29) measured the factor structure of this questionnaire and showed that it has high convergent validity, as well as internal compatibility and test-retest reliability. Also, they reported the Cronbach’s alpha of this questionnaire equal to 0.85 (29). Alizadehgoradel et al. (30) reported good internal consistency of this questionnaire (0.88) for people with methamphetamine use disorder.
3.3. Statistical Analysis
We used SPSS version 24 to analyze the collected data. To measure the effectiveness of each treatment and their combined effects in the improvement of negative emotions and craving, a mixed ANOVA was used to analyze the data, with time as the within-group factor (pretest, posttest, follow-up) and group (tDCS, MBSAT, PIN-CODES, sham) as the between-subject factor. The Bonferroni test was also used for paired comparisons.
4. Results
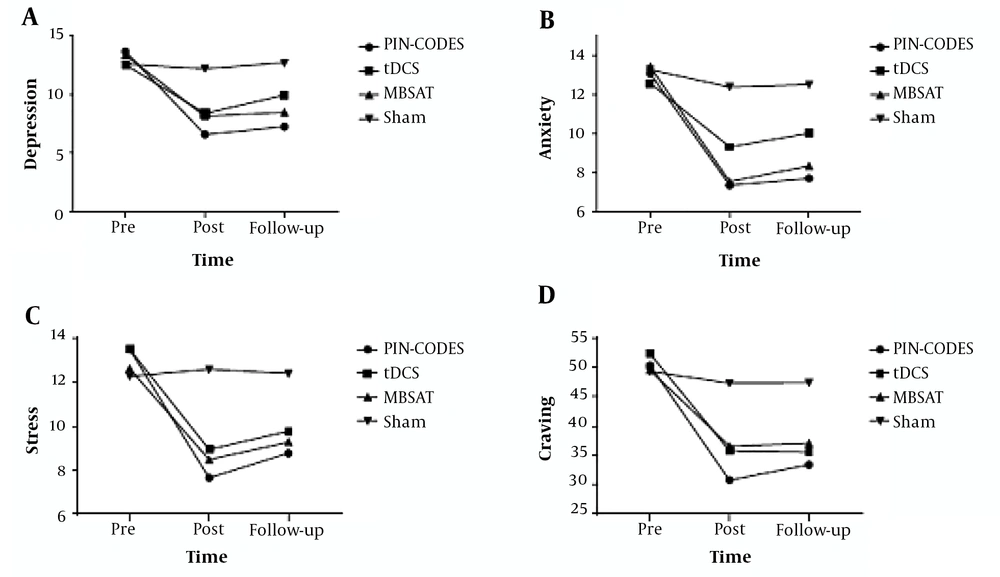
In terms of the demographic characteristics of the adolescent participants with methamphetamine dependence, there was no difference between the groups (mean age = 19.46, SD = 1.15) (Table 2). Adolescents in the present study showed the tolerable potential to electrical stimulation and did not report significant side effects (Table 3). The data overview, including the means and standard deviations of negative emotions and craving, is presented in Figure 2. The correlations between the craving reduction rate after the intervention and the rates of change from pre-to-post intervention in negative emotions of the tDCS + MBSAT group are summarized in Table 4.
| tDCS | MBSAT | PIN-CODES | Sham | P-Value | |
|---|---|---|---|---|---|
| Sample size, N | 16 | 15 | 17 | 16 | |
| Age, mean (SD) | 19.43 (1.20) | 19.46 (1.12) | 19.52 (1.23) | 19.43 (1.15) | 0.995 |
| Sex | |||||
| Male | 16 | 15 | 17 | 16 | |
| Female | 0 | 0 | 0 | 0 | |
| Marital status | 0.861 | ||||
| Single | 11 | 11 | 12 | 13 | |
| Married | 5 | 4 | 5 | 3 | |
| Length of methamphetamine use, mean (SD) | 3.06 (0.77) | 3.13 (1.06) | 2.88 (0.99) | 2.87 (0.89) | 0.812 |
| Age of onset of substance use, mean (SD) | 15.62 (1.40) | 15.26 (2.15) | 15.88 (1.61) | 15.00 (2.12) | 0.541 |
| Substance use by family members | 0.556 | ||||
| Yes | 8 | 6 | 11 | 9 | |
| No | 8 | 9 | 6 | 7 | |
| Education | 0.633 | ||||
| Under the diploma | 10 | 9 | 8 | 11 | |
| Diploma | 6 | 6 | 9 | 5 |
| Tdcs Session | Itching Sensation | Burning Sensation | Pain | Tingling | Fatigue | Trouble Concentrating |
|---|---|---|---|---|---|---|
| PIN-CODES | 9 | 10 | 6 | 12 | 5 | 2 |
| Anodal l-DLPFC | 8 | 8 | 5 | 12 | 6 | 2 |
| Sham tDCS | 6 | 5 | 3 | 6 | 2 | 1 |
| χ2 (active vs. sham) | 3.39 | 3.20 | 1.57 | 4.43 | 1.36 | 0.01 |
| P | 0.75 | 0.78 | 0.95 | 0.61 | 0.85 | 1.00 |
aSignificant results at the 0.01 level.
bSignificant results at the 0.05 level.
The results for negative emotions (in all components) showed that the main effect of the group was significant. The Bonferroni correction post hoc analysis further showed that the combined treatment was more beneficial for negative emotions and craving compared to the sham and other treatments (Table 5). For the anxiety (P pin-codes < 0.002), depression (P pin-codes < 0.002), stress (P pin-codes < 0.015) and DDQ (P pin-codes < 0.001) score combined group significantly improved scores of participants compared to the sham group. The results of between-group differences showed that all three therapies were effective when compared to the control group although the combination therapy was more effective than the other two methods. We also used the Pearson correlation coefficient to investigate the relationship between the percentage change of craving for substance abuse from pretest to posttest and the percentage of negative emotions. The results showed a significant correlation of reduced craving with anxiety and depression in the PIN-CODES group (Table 4).
| Variable | Outcome Measures | Source | df | f | P | eta2 | Pairwise Comparisons (Bonferroni) |
|---|---|---|---|---|---|---|---|
| DAAS21 | Depression | Time | 2,120 | 119.72 | 0.001 | 0.66 | PINCODES > Sham (P < 0.002); tDCS > Sham (P < 0.029); MBSAT > Sham (P < 0.029) |
| Group | 3,60 | 19.80 | 0.001 | 0.49 | |||
| Time*group | 6,120 | 15.32 | 0.001 | 0.43 | |||
| Anxiety | Time | 2,120 | 98.49 | 0.001 | 0.62 | PINCODES > Sham (P < 0.012); MBSAT > Sham (P < 0.045) | |
| Group | 3,60 | 10.90 | 0.002 | 0.35 | |||
| Time*group | 6,120 | 9.39 | 0.001 | 0.32 | |||
| Stress | Time | 2,120 | 76.03 | 0.001 | 0.55 | PINCODES > Sham (P < 0.015); MBSAT > Sham (P < 0.034) | |
| Group | 3,60 | 11.22 | 0.001 | 0.35 | |||
| Time*group | 6,120 | 11.88 | 0.001 | 0.37 | |||
| Craving | DDQ | Time | 2,120 | 121.66 | 0.001 | 0.67 | PINCODES > Sham (P < 0.001); tDCS > Sham (P < 0.036); MBSAT > Sham (P < 0.027) |
| Group | 3,60 | 11.78 | 0.001 | 0.39 | |||
| Time*group | 6,120 | 12.17 | 0.001 | 0.37 |
5. Discussion
The current study aimed to examine the effects of the electrical stimulation of DLPFC combined with mindfulness-based substance abuse treatment on negative emotions and craving in adolescents with methamphetamine use disorder. Our study showed that this intervention (combination therapy) significantly reduced craving and improved negative emotions. In addition, intergroup differences indicated that significant changes occurred in all three therapies from pretest to posttest and follow-up. New psychological therapies, such as Mindfulness-based interventions and tDCS, attempt to target neurocognitive mechanisms of addiction and have shown promising results in patients with substance use disorders (31). On the other hand, new studies indicate that tDCS would be more useful if used as a complementary technique (32). The combined effects of tDCS + MBSAT affirm that tDCS has more beneficial and lasting therapeutic effects when combined with psychological interventions, which is compatible with recent studies supporting the additive effect of tDCS when combined with other psychological interventions (22-26).
In this regard, our results showed that PIN-CODES, as a new treatment approach for the treatment of drug addiction, is more beneficial for improving negative emotions and craving in adolescents with methamphetamine use disorders. To the best of our opinion, this is the first attempt to combine psychotherapy with tDCS to improve negative emotions and cravings in a randomized trial. In most addiction studies using tDCS alone, positive effects of TDCS are limited to improving the cognitive function of substance abusers (33). The combined effects of the PIN-CODES therapy approach confirm the incremental effects of electrical stimulation therapy in combination with other psychological interventions (32).
As known, PIN-CODES can better determine the mechanism of mindfulness. Previous studies showed that in clinical populations, negative emotions are enhanced by tDCS (20). Since these negative emotions are also targeted by mindfulness (33, 34), the greater reduction in craving and improvement in negative emotions in the PIN-CODES group may be related to the direct involvement of enhancing negative emotions.
Modern treatments of substance abuse disorder emphasize the non-invasive neuromodulation of prefrontal regions, especially the dorsolateral prefrontal cortex (DLPFC). The DLPFC is mainly involved in executive functioning (35) and emotion regulation (36). Recent studies showed that tDCS as a non-invasive brain stimulation technique is a promising tool for the treatment of drug addiction (30, 37). The non-invasive stimulation of DLPFC may be linked to alterations in negative emotions (38). The results show that promoting emotion regulation and improving ACC/mPFC brain activity can help with addiction prevention and treatment (39).
In sum, our findings in the present study are significant clinical applications of combined tDCS+MBSAT (PIN-CODES) in methamphetamine use disorder. The first application of the present study is to suggest a PIN-CODES protocol (tDCS with mindfulness) for the treatment of persons with substance use disorders. Future studies are recommended to investigate the effects of tDCS in combination with other psychological interventions such as cognitive-behavioral treatments. Despite promising results for the treatment of drug abuse using the PIN-CODES therapy approach, some limitations need to be considered when interpreting our findings. The follow-up stage in this study lasted one month; so, to evaluate the long-term effects of treatment, it is suggested that studies with longer follow-up periods be conducted. The lack of a larger sample size was another limitation. It is suggested that future research replicate our interventions in larger samples. Finally, the present study was the lack of a instrument such as fMRI for measuring brain changes after the intervention; thus, future research is proposed to use brain imaging techniques to better describe brain changes.