1. Background
Methamphetamine, popularly known as glass in Iran, is a stimulant that causes brain neurotoxicity and structural damages (1, 2). Methamphetamine is biochemically similar to epinephrine and dopamine, and its abuse leads to personal dependence and neuro-psychological impairments. A variety of cognitive and behavioral dysfunctions can occur after substance use among which, changes in memory, attention, and decision-making (3, 4), episodes of hallucination and paranoid psychosis, and even incidence of violence or criminal behaviors are noticeable (1, 5).
The chronic use of MA imposes some deficits by its toxicity on the brain structure. It can reduce the gray matter in the cingulate, limbic, and paralimbic cortices, and change cortical activities (3, 6). Long-term MA use specifically affects dopaminergic structures such as putamen, caudate, and striatum (7, 8) and consequently alters brain neurotransmitter substances (9, 10). Brain electrical activity may be disrupted by the chronic use of MA, and cortico-cortical neural connections may change, as well (11, 12).
Brain Functional Connectivity (FC) is referred to as the synchronization in functional activity between different neuronal populations (12). Electroencephalographic coherence is a tool that represents brain functional connectivity (13, 14). It is a quantitative electroencephalographic index to calculate the synchronization of neuronal oscillation and reflects the integration and dependence of activation in different parts of the brain (15). Higher levels of coherence reflect functional dependence and integration of electrical activation between two regions, while lower coherence values refer to the independence of the neural population in electrical oscillation. As mentioned before, chronic exposure to MA may damage cortico-cortical network connection and can alter brain functional connectivity that has an important role in information processing. Therefore, the investigation of coherence as an index of functional connectivity could be important for the deep understanding of brain features and performance after a chronic use of MA.
Despite the importance of methamphetamine effects on brain function, a few studies have been done to investigate brain functional connectivity. These studies have examined entropy and small-world networks (12) as nonlinear indicators of functional connectivity. They show changes in the nonlinear parameter in methamphetamine users, especially in slow-wave frequency bands, even during abstinence (7, 12). However, there is a type of discrepancy and complexity in these studies and thus, EEG connectivity coherence has not been well understood.
On the other hand, the composition of synthetic drugs, especially in developing countries and the Middle East, may induce different chemical properties (11). It implies that obtaining more information about EEG coherence could be helpful for a deeper understanding of brain pathologies in MA users and may assist in adopting appropriate medications and neurorehabilitation strategies such as EEG coherency biofeedback.
2. Objectives
We conducted a study to investigate the effect of chronic MA use on EEG coherence in the abstinence period.
3. Materials and Methods
3.1. Sampling
A cross-sectional study was conducted on a sample of 18 methamphetamine users and 18 healthy volunteers (right-handed males aged 20-45 years). Patients were hospitalized at Razi Mental hospital of Tabriz, Iran, in terms of psychiatric emergency conditions. Healthy non-users were recruited among outpatients. Right-handed males with a long-term history of methamphetamine use (at least one year) and positive methamphetamine screening tests in the past month were enrolled in the study. The exclusion criteria were a positive history of neurological disorders, seizure, and cognitive impairment (based on psychiatric history) and high doses or long-term intake (more than one week) of chlorpromazine, sodium valproate, lamotrigine, topiramate, and benzodiazepines. The maximum acceptable doses of the drugs were 25 mg chlorpromazine, 25 mg lamotrigine, 250 mg sodium valproate, 25 mg topiramate, 1 mg lorazepam, 0.5 mg alprazolam, and 0.25 mg clonazepam. The other exclusion criteria were a positive morphine screening test in the last 90 days and a history of any psychiatric disorder (for the control group).
3.2. EEG Recording
Electroencephalography signal acquisition was performed using a 19-channel Ag/C1 electrode with an EEG amplifier (Mitsar, Russia) according to the standard 10-20 system. The linked ear reference was used for signal recording and electrode impedance was kept below 5 kΩ during EEG acquisition. The sampling rate of the signal was 500 Hz, and bandpass filter sets were in the range of 0.1-30 Hz. Brain electroencephalography was recorded in eyes-closed and eyes-open resting states for 10 minutes in both groups. The EEG recording in methamphetamine users was done after the first week of their admission before receiving high doses of psychiatric medications (Table 1).
| Methamphetamine consumption | Dose |
|---|---|
| Average dose of methamphetamine in the last six months, gr | 1.5 |
| Dose based on weight, milligram | 22.3 |
| Minimum dosage, gr | 3.5 |
| Average duration of MA use, year | 5 |
| Abstinent period, day | 7-9 |
3.3. Statistical Analysis
The analysis for coherence was performed between twin electrodes, and comparisons were made between temporal, occipital, frontal, and parietal lobes. Frequency spectrum analysis was conducted using Neuroguide software, and all data were analyzed by SPSS version 17. Descriptive statistical methods and repeated-measures ANOVA and t-test were used whenever needed. P < 0.05 was considered significant.
3.4. Ethics
This study was approved by a Regional Ethics Committee affiliated to Tabriz University of Medical Sciences under code TBZMED.REC.1394.26. The patients were appropriately treated and no intervention was imposed.
4. Results
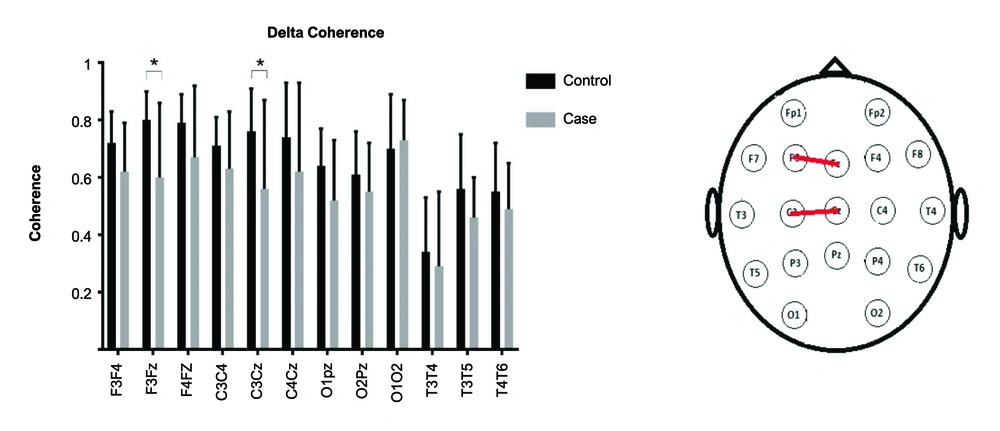
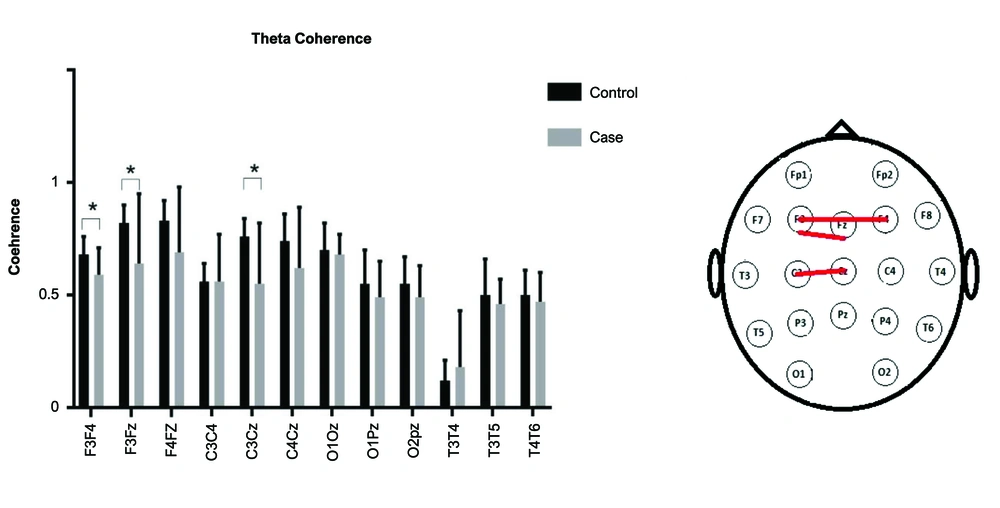
In this study, coherence was investigated in frontal, temporal, parietal, and occipital lobes. Table 2 shows the electrodes used for coherence analysis. Coherence indices were obtained for delta and theta frequency bands based on equation 1. The independent t-test showed no difference in terms of age between the two groups (P = 0.37).
| Occipital lobe | Parietal lobe | Temporal lobe | Frontal lobe | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O2- | O1- | O1- | C4- | C3- | C3- | T4- | T3- | T5- | F4- | F3- | F3- |
| Pz | Pz | O2 | Cz | Cz | C4 | T6 | T5 | T6 | Fz | Fz | F4 |
In this equation, Gxy(f) is the cross-spectral density between x and y, and Gxx(f) and Gyy(f) stand for the auto-spectral density of x and y, respectively (16).
A repeated-measures ANOVA was used. As the sphericity assumption was violated, Greenhouse-Geisser's F was reported.The interaction of Group (2)× Lobe (4)× Band (2)× Electrode (3) was significant [F(4, 129) = 2.53, P = 0.04]. Other interactions with group were non-significant (P values > 0.5). The post hoc independent t-test was used to compare the coherence of electrode pairs for delta and theta bands (Figures 1 and 2). In the delta band, the electrode pairs of F3Fz [t(18) = -2.8, P = 0.01] and C3Cz [t(20) = -2.2, P = 0.03] were significantly different between the two groups. In the theta band, the electrode pairs of F3F4 [t(29) = -2.3, P = 0.03], F3Fz [t(16) = -2.2, P = 0.04], and C3Cz [t(16) = -2.9 , P = 0.01] were significantly different between the two groups.
5. Discussion
In this study, we investigated the effect of methamphetamine use on brain connectivity by assessing the coherency index of qEEG in recently abstinent users. The results revealed an attenuation in slow-wave (delta and theta bands) coherence after the long-term use of MA. The cortico-cortical delta band coherency in the left frontoparietal lobe and theta band in the left inter and intra-hemispheric regions were diminished in users.
Changes in functional connectivity in the MA group, as revealed in the current study, are compatible with the results of research by Ahmadlou et al. (12). They showed some changes in the topology of functional brain connectivity in chronic MA users after abstinence. These changes were especially observed in gamma and delta bands according to the data from the Small-Word network (nonlinear index of coherency). They interpreted the observed result as the cause of cognitive deficit in MA users (12), which also was observed in illnesses accompanied by cognitive dysfunction such as Alzheimer's disease, schizophrenia, and depression (7, 17).
In another study by Yun et al., functional connectivity and brain complexity of different sites of the brain were investigated by assessing the entropy index. This index displays dynamic interplay between the two sites of the cortex and the amount of integration or segregation between neuronal populations (14). They observed a significant attenuation in brain complexity in methamphetamine users in frontal and temporal regions, which is compatible with our findings. They implied that the long-lasting toxic effect of MA on functional and structural circuits of the brain changes the brain regional connectivity of the cortex. They also proposed that the duration and the amount of exposure to MA could affect the results (7).
Modification in the brain coherence index in this study could be due to the toxic effect of MA on cell activity, as frequently reported in previous studies. This can consequently reduce the activation of neuronal populations (18), leading to neural dysfunction (19), damage to the metabolism of glucose (20), and decreases in regional cerebral blood flow (21).
In addition, changes in brain electrical activity could be a brain response to methamphetamine deprivation or a way to compensate for it. It seems that the neural system tries to maintain its homeostasis after withdrawal and consequently changes the brain’s electrical activity (12, 22).
Alterations in the coherence index in the present study were seen in the frequency domains of slow waves (delta and theta frequency bands). As considered before, the resting-state slow-wave oscillation could be somehow related to the brain reward system (22). This circuit is mediated by dopaminergic neurotransmitters. Therefore, any change in the reward circuit could be associated with the modulation of slow-wave oscillation and EEG (23). Studies show that substance use is associated with releasing dopamine in the nucleus accumbens, which activates the reward circuits (24). However, the long-term use of methamphetamine can cause marked structural and physiological changes in brain dopaminergic cells and neurotransmitters (8), particularly the metabolism of dopamine systems. Thus, the potential toxicity effect of methamphetamine on dopaminergic and noradrenaline neurotransmitters (22) could be considered as an important factor in the local reduction of cortical slow-wave activity and coherence in different areas of the cortex.
On the other hand, it has been shown that the reward-related release of dopaminergic neurotransmitters in opiate and heroin users is associated with a decrease in slow-wave oscillation, and thus, some increases in delta and theta waves are expected after abstinence (25, 26). However, studies on the electrophysiological features of the brain in substance addiction revealed a complex picture of EEG activation in different stages of drug administration and quitting. For example, studies reported an increase in delta and theta band oscillation after MA abstinence (27) and caffeine withdrawal (28). Abstinence from cocaine showed a reduction in slow-wave coherence between brain inter-hemispheres (29, 30). In this study, we also found a decrease in the coherence of slow waves, which was contrary to theoretical expectations. One explanation for these inconsistencies is the severe drug toxicity damages to brain biochemistry and functions. Studies revealed that even the cognitive ability of the brain, such as emotion and motivation, is fundamentally damaged in chronic MA users (6). The amount of destruction to the brain structure and function could be related to the amount of exposure, drug composition, and the way of drug intake. As a result, such complexity in the results of research in the field of substance abuse is not unexpected. However, it should be considered that people with a tendency to addiction and drug abuse may have had some underlying genetic differences in their brain waves from the beginning, like what is seen in Reward Deficiency Syndrome (RDS). People with RDS are basically at a greater risk of getting a reward through abnormal ways, such as drug abuse (31). Thus, it seems that some longitudinal studies are needed for a better understanding.
5.1. Conclusion
Modulation of the coherence index in MA users in this study indicates that, as a representative for brain connectivity, it could be sensitive to changes made by MA. The coherence index decreased in chronic MA users, especially in slow waves. This result might be an indicator of segregation between different neuronal populations, which, by itself, could be the cause of deficits and neuronal dysfunction.
5.2. Limitation
In this study, due to limited equipment in the psychiatric center, 19-channel electroencephalography was used. More electrode numbers are preferred for measuring the coherence index. Subjects who were included in this study were all admitted to a psychiatric hospital due to emergency conditions. This, in addition to being a limitation of the present study, may reduce the possibility of its generalizability to consumers who do not exhibit such a condition.