1. Background
Opioid misuse is a worldwide concern across the entire lifespan in the general population. There has been a permanent increase in opioid misuse over the last decade that has resulted in considerable harm to individuals, families, and the wider community (1). It is estimated that 53.4 million people have used opioids at least once (2), and 40.5 million had opioid dependence in 2017, globally (3). Opioid use is associated with the highest proportion of the burden of disease among all illicit drug use disorders (2, 4).
The epidemic of opioid use disorder in adolescents and young adults is a growing problem that can lead to psychosocial impairment. Its medical morbidity includes criminal justice involvement, school dropout, unemployment, co-occurring psychiatric disorders, hepatitis C virus, human immunodeficiency virus, injection site infection, and the worst of all, premature death associated with overdose (5). An earlier age of opioid use onset is associated with worsening consequences. Adolescents who were primarily using opioids had an earlier age of onset of any substance use than those who were current users of cannabis or alcohol (6).
An estimated 22% of adolescents in Iran have opiate misuse (7). There are challenges in the provision of opioid dependence treatment for adolescents and youth with opiate dependence. Patient engagement is challenging, and family and community involvement are necessary (5). Opioid agonist maintenance treatment is the standard treatment for adults with opioid dependence (8). However, adolescents with opiate dependence have limited access to opioid agonist medications (9).
Buprenorphine, a partial opioid agonist, and partial antagonist can be used both as maintenance and withdrawal treatment. The US Food and Drug Administration (FDA) approved buprenorphine for the treatment of adolescents with opioid dependence in 2003 (10). Buprenorphine prescribing is a viable component of standard adolescent opioid dependence treatment. Buprenorphine, unlike methadone, is recommended for younger patients (11). Up to now, only a few randomized controlled trials have provided data using buprenorphine to treat adolescents and young adults with opiate use disorders (12-14).
Clonidine, an alpha-agonist agent, has been used for the treatment of opioid withdrawal (15). Clonidine provides dose-related reductions in blood pressure and heart rate. Furthermore, clonidine reduces the autonomic signs of opioid withdrawal and suppresses the subjective discomfort of it (16). Studies have confirmed the effectiveness of clonidine in the management of withdrawal symptoms of opiate dependence (17-20).
2. Objectives
Despite many studies showing the effectiveness of buprenorphine and clonidine in adults, there is a lack of studies to evaluate compare these medicines in adolescents. This study aimed to compare the efficacy of clonidine and buprenorphine for inpatient medically-assisted withdrawal of adolescents with opioid dependence aged 12 and 16 years.
3. Materials and Methods
3.1. Participants
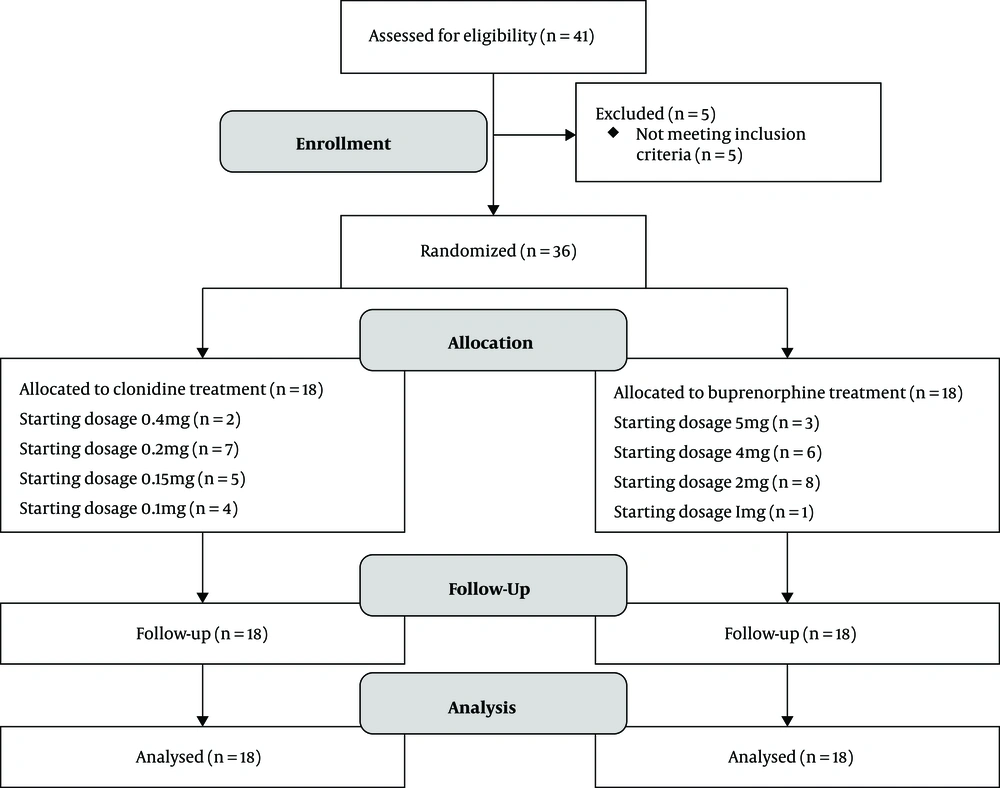
A total of 38 patients were enrolled in this open-label randomized trial with convenience sampling. The research was done in Ali-Ebne-Abitaleb hospital in Zahedan, southeast of Iran. The participants were recruited from March 2018 to December 2019. Two psychiatrists diagnosed patients. The sample size was calculated based on the study by Motamed et al. (21) A block randomization scheme with a block size of six was used to assign participants to clonidine or buprenorphine groups. All participants received inpatient, medically-assisted withdrawal with buprenorphine or clonidine. Study assessments were completed on days one, two, three, seven, and 14 (Figure 1).
Inclusion and exclusion criteria: Participants included self-referred adolescents who met ICD-10 criteria for opioid dependence. All the participants with opiate use disorder aged 12 to 16 years were included in the study. The patients were excluded if they had other psychiatric disorders or known sensitivity to study medications.
Ethical consideration: The Ethics Committee of Zahedan University of Medical Sciences approved the study (IR.ZAUMS.REC.1397.249), and all procedures were submitted to the Iranian Registry of Clinical Trials (IRCT20150926024209N6). The participants' parents or legal guardians provided informed consent, and the participants provided informed assent to participate in the study.
3.2. Interventions
In the buprenorphine group, medicine started on the first day of hospitalization. Therefore, patients did not experience the withdrawal symptoms on day one, but medicine for the clonidine group started when their withdrawal symptoms appeared. Buprenorphine group: We applied buprenorphine according to the current community treatment plan, including baseline screening and follow-up on days two, three, seven, and 14. Participants in this group were given buprenorphine at a dose of 1 - 6 mg. The baseline in this group was the first day of hospitalization.
Clonidine group: We applied clonidine according to the current community treatment plan, including baseline screening and follow-up on days two, three, seven, and 14. Participants in this group received clonidine 0.1 - 0.4 mg. One participant received loperamide (2 mg), and two patients received hydroxyzine (10 - 50 mg) and ibuprofen (100 - 800 mg). The baseline was the first day of withdrawal symptoms appearance.
3.3. Measures
The Clinical Opiate Withdrawal Scale (COWS), a clinician-administered scale with 11 items, was used to assess the patient's level of opiate withdrawal in both inpatient and outpatient settings. This scale can be used to monitor withdrawal symptoms over time (22). The COWS has shown its clinical usefulness in opioid withdrawal management studies among both adult (23, 24) and adolescent (25) patients in Iran.
3.4. Statistical Analysis
Data analysis was done by the IBM SPSS software, version 22 (IBM Corp). The Mann-Whitney U test and Chi-square test were used to compare the demographic data. The statistical analysis of data was done with nonparametric tests, including Fisher's exact test to compare the effectiveness of clonidine and buprenorphine. The post hoc analysis with Wilcoxon signed-rank test and Bonferroni correction was applied. The Kruskal Wallis H test was employed to compare the efficacy of two treatments. All graphs were drawn by GraphPad Prism 8.
4. Results
4.1. Sociodemographic Characteristics
The results showed that the mean age of the participants was 13.66 ± 1.65 years, and 14 participants were female. Age (P = 0.15) and gender (P = 0.49) were matched between the two groups. The mean duration of regular opioid use was 34.11 ± 32.74 months. The sociodemographic characteristics of the groups are summarized in Table 1. The length of hospital stay in the whole sample was 13.22 (± 4.64), and there was no significant difference between the clonidine (13.16 ± 5.30) and buprenorphine (13.25 ± 4.53) groups (P > 0.05).
| Variables | Clonidine Group (N = 18) | Buprenorphine Group (N = 18) |
|---|---|---|
| Age (y) | 13.33 ± 1.41 | 14.00 ± 1.45 |
| Gender | ||
| Female | 6 (33.3%) | 8 (44.4%) |
| Male | 12 (66.7%) | 10 (55.6%) |
| Duration of regular opioid use (mo) | 36.66 ± 40.63 | 31.55 ± 23.28 |
| History of medically-assisted withdrawal treatment | ||
| Yes | 4 (22.2%) | 7 (38.9%) |
| No | 14 (77.8%) | 11 (61.1%) |
| Type of substance abuse | ||
| Heroin | 13 (72.22%) | 16 (88.88%) |
| Opium | 6 (33.33%) | 5 (27.77%) |
| Methamphetamine | 12 (66.66%) | 13 (72.22%) |
| Tramadol | 1 (5.55%) | 0 |
aValues are expressed as mean ± SD or No (%).
4.2. Effectiveness of Clonidine-Assisted Withdrawal Treatment
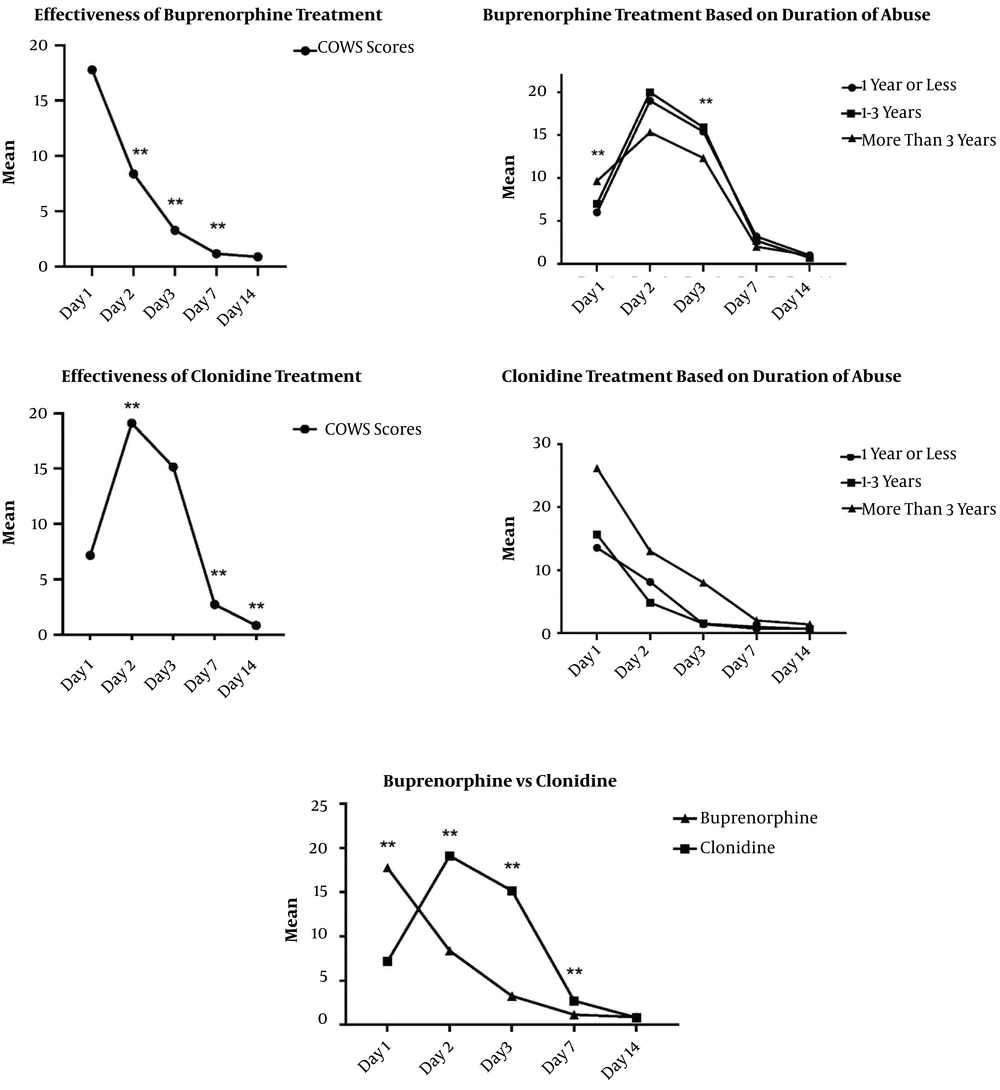
The Friedman test was used to compare the COWS scores of clonidine treatment. There was a significant difference between the follow-up sessions [χ2(4) = 60.64, P < 0.001]. Post hoc analysis with the Wilcoxon signed-rank test was conducted with Bonferroni correction, resulting in a significance level set at P < 0.01. There were significant differences between day one and day two (z = -3.58, P < 0.01). There was no significant difference between day two and day three (z = -2.44, P = 0.015). However, there was significant differences between day three and seven, as well as day seven and day 14 (z = -3.72, P < 0.01 and z = -2.69, P < 0.01). The Kruskal-Wallis H test showed no statistically significant difference in the effectiveness of clonidine treatment between patients with different durations of regular opioid use (Figure 2).
4.3. Effectiveness of Buprenorphine-Assisted Withdrawal Treatment
The results of the Friedman test showed significant differences between the follow-up sessions [χ2(4) = 60.89, P < 0.001]. Post hoc analysis with the Wilcoxon signed-rank test was conducted with Bonferroni correction, resulting in a significance level set at P < 0.01. There were significant differences between day one and day two (z = -3.37, P < 0.01), day two and day three (z = -3.73, P < 0.01), and day three and day seven (z = -1.63, P < 0.01). There was no significant difference between day seven and day 14 (z = -1.63, P = 0.102) (Figure 2). The Kruskal-Wallis H test showed a statistical difference in baseline and day three between patients with different durations of regular opioid use.
4.4. Comparing the Effectiveness of Clonidine and Buprenorphine Opioid Withdrawal Treatments
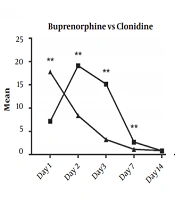
The Kruskal-Wallis H test showed a statistically significant difference between the COWS scores in baseline [χ2(1) = 18.17, P < 0.001], day two [χ2(1) = 17.64, P < 0.001], day three [χ2(1) = 21.03, P < 0.001], and day seven [χ2(1) = 8.93, P < 0.001]. There was no statistical difference between the COWS scores on day fourteen [χ2(1) = 0.0, P = 0.98] (Figure 2/Table 2).
| Day of Treatment | Buprenorphine Group (N = 18) | Clonidine Group (N = 18) | P-Value |
|---|---|---|---|
| Day 1 | 17.77 ± 7.16 | 7.16 ± 4.73 | < 0.01 |
| Day 2 | 8.38 ± 7.38 | 19.11 ± 5.23 | < 0.01 |
| Day 3 | 3.27 ± 4.86 | 15.16 ± 5.63 | < 0.01 |
| Day 7 | 1.16 ± 1.29 | 2.72 ± 1.99 | < 0.01 |
| Day 14 | 0.88 ± 0.67 | 0.83 ± 0.51 | 0.98 |
aValues are expressed as mean± SD.
5. Discussion
Various medications have been used for the treatment of adolescents with different psychiatric disorders. In contrast, medications have been infrequently used for treating substance use disorders among adolescents. Because of the nature and pharmacologic properties of opiate drugs, adolescents who are physically dependent on opioids will experience a severe physical withdrawal syndrome if they abruptly discontinue their opiate use, presenting a major obstacle to the treatment of opioid dependence (13).
This study compared the effectiveness of buprenorphine versus clonidine treatment for the detoxification of adolescences with opioid dependence. For this purpose, 36 adolescences between 12 to 16-years-old were assigned to two groups to receive either buprenorphine or clonidine. The COWS scores were monitored on days one, two, three, seven, and 14. The findings showed that both treatments were effective, but buprenorphine was superior to clonidine between days two to seven. However, after day seven, the COWS scores were comparable between both groups, and the subjects of both groups on day 14 were mostly withdrawal-free.
There was no difference in the duration of hospitalization between the two groups. Furthermore, there was no difference between both treatments based on the duration of opiate abuse; however, patients with a longer duration of abuse in the clonidine group showed more withdrawal symptoms on day one and day three.
The effectiveness of buprenorphine and clonidine in adolescents and young adults was shown in other studies. Levy et al. reported that buprenorphine is as effective as high-dose methadone in the treatment of adolescents and it may be better suited for the treatment of younger patients (26). Marsch et al., in a study of 36 adolescents aged 13 - 18 years, compared a 28-day outpatient treatment with either buprenorphine or clonidine and showed that a greater percentage of adolescents who received buprenorphine were retained in treatment relative to those who received clonidine. Patients in both groups reported the alleviation of withdrawal symptoms. However, the buprenorphine group generally reported more positive effects of the medication (13). In a study by Motamed et al., 36 adolescents (aged 13 - 18) with opioid dependence received a 28-day, outpatient, medication-assisted withdrawal with partial opioid-agonist buprenorphine or clonidine. Patients in this study also took behavioral counseling. Both heroin-dependence and prescription opioid-dependence adolescents who received buprenorphine experienced notably better treatment outcomes than those who received clonidine (21). A clinical review concluded that buprenorphine is more effective than abstinence-based treatment like clonidine, and physicians should recommend buprenorphine treatment over abstinence-based treatment, and for adolescents, treatment retention should take precedence over other clinical considerations (27).
The development of effective treatment and safe detoxification for opioid dependence in adolescents is of great importance. Studies regularly compared the efficacy of buprenorphine and clonidine in adults with opiate dependence (28-30). These studies showed that buprenorphine demonstrated to be better than clonidine in controlling opioid withdrawal. While clonidine was long used as the primary detoxification medication, buprenorphine is now more routinely used because it is physiologically directed toward opiate receptors, and that is why it is more effective in relieving the symptoms of withdrawal (5). Among pharmacological agents that have been used as detoxification agents to reduce withdrawal symptoms, buprenorphine has some advantages for adolescents because of the absence of long-term complications (26). In this study, it was observed that agonist treatment with buprenorphine was superior to clonidine in controlling opioid withdrawal during the first few days of detoxification.
In conclusion, buprenorphine treatment was found to be more effective than clonidine in controlling opioid withdrawal. However, it lost its superiority towards the end of the follow-up. It seems that clonidine could be a good alternative to buprenorphine in detoxification. Given these findings, the debate on the superiority of treatments makes little sense. In the era of individualized medicine, there is no debate against having multiple evidence-based treatment options where individual planning can be tailored to patient risks and needs, instead of using only one of the treatments, it is better to develop a combination protocol of both methods. (31).
The current study has some limitations. As it was an inpatient study, the generalization of the findings to outpatient treatments must be with caution. This study focused on outcomes during detoxification, so the patients were not followed to examine the effect of the two treatments on maintenance or relapse of opioid addiction. It was an open-label study, and blinding was not done. The type of opioid use was not specified, although it is expected that the onset of withdrawal symptoms was earlier in injecting consumers than in oral consumers. Few patients in the clonidine group received other medicines (ibuprofen, loperamide, and ibuprofen), which can have affected the results.