1. Background
Oral cancers are the sixth most common cancer in the world. Squamous cell carcinoma (SCC) accounts for approximately 60% of oral malignancies (1) and 38% of all head and neck tumors (2). It begins with a precancerous process in which the foci of abnormal cells are selectively from the squamous epithelium. Precancerous to malignant transmission occurs before attack on connective tissue when tumor cells destroy the basal layer (BL) (3). Squamous cell carcinoma is graded based on the similarity of the malignant cell to the primary tissue and creatinine content (4). The prognosis of SCC is determined based on the clinical pathology staging system (TNM), anatomical status, and mitotic activity (1). Local invasion and widespread spread to the lymph nodes lead to an unfavorable prognosis of SCC (5). Due to significant differences in survival rates, biological markers are needed for a more accurate prognosis (1). In fact, accurate detection of biological markers helps to better identify tumor behavior and its metastatic potential (2). Verrucous carcinoma (VC), known as Ackerman’s tumor, is a rare form of low-grade SCC. It is the most common injury in the oral cavity with unknown etiology. However, there is speculation that the human papillomavirus (HPV), ultraviolet (UV) radiation, and chronic tobacco use may be the cause (6). In 1 - 10% of cases, VC is associated with SCC. Also, it is rarely associated with metastasis (4). VC is characterized by nuclear growth and local invasion without metastasis and may grow in surrounding tissues (6). Matrix metalloproteinases (MMPs) play an important role in tumor biology, including initial carcinogenesis caused by tumor growth to extracellular matrix (ECM) to degradation and invasion (7). Matrix metalloproteinases 2 and 9 can predict the potential for invasion and metastasis and determine the prognosis of the tumor (5). Matrix metalloproteinases are a family of zinc-dependent proteinases that are secreted in the form of proenzymes and require proteolytic enzymes to be activated (1). Matrix metalloproteinases 2 and 9 are the largest members of the enzyme family, consisting of five groups. These enzymes are encoded by two separate genes (1). Matrix metalloproteinase-9 is physiologically active in polymorphonuclear leukocytes (PMNs) and helps heal wounds and inflammation (8). Matrix metalloproteinases 2 and 9 are known as collagenase IV, which can also destroy gelatin and collagen IV, V, VII, IX, X (7). In the early 1980s, it was discovered that degradation of type IV collagen of the basal layer was critical for melanoma invasion (9). Matrix metalloproteinases 9 and 2 have been considered in studies due to their roles in the development of dysplasia to malignancy (1). They can be used as markers to detect SCC, VC. Matrix metalloproteinase-9 stimulates the secretion of factors such as transforming growth factor-β and vascular endothelial growth factor, which leads to tumor spread and angiogenesis (8). Expressions of MMPs 2 and 9 are indicators in determining prognosis and survival (7). This paper uses immunohistochemistry, which allows direct evaluation of morphological data and can be applied to sections of paraffin tissue commonly used in diagnosis (9).
2. Objectives
The aim of this study was to evaluate the immunohistochemistry of MMPs 2 and 9 in oral SCC (OSCC) and VC.
3. Patients and Methods
3.1. Tissue Samples and Patients
In this study, 38 medical records of 20 patients with OSCC and 18 patients with VC were collected from the archives of the pathology department. Participants were selected from patients who had not received any medical intervention before surgery. Only medical records containing paraffin blocks were selected and re-examined by a pathologist. Once approved, the appropriate location for the IHC was determined on the slides. These blocks had the largest and most suitable tissue volume for immunohistochemistry. Positive control samples, including placental tissue blocks for MMP-9 and normal labial mucosal blocks for MMP-2 were prepared.
3.2. Immunohistochemistry
After re-examination of the specimens by a pathologist and marking of appropriate sites for the expression of MMP-9 (NeoMarkers, California, USA) and MMP-2 (Novocastra, Newcastle, UK), two 3-μm thick sections of each paraffin block was prepared. First, paraffin blocks with a thickness of 3 μm were kept at room temperature for 24 hours. Then, they were deparaffinized in xylene to perform the staining steps according to the manufacturer’s instructions: For antigen retrieval, the blocks were first incubated in citrate solution for 35 minutes in the microwave. They were then washed with distilled water and placed in a humid environment. Next, they were lacated in solutions containing peroxidase, MMPs 2 and 9 antibodies, link, streptavidin, chromogen, and hematoxylin. Between each stage, the samples were placed in pure alcohol for five minutes and then mounted by Entellan. The stained slides were examined with an optical microscope at 400X magnification. To ensure staining accuracy, positive control samples, including placental tissue blocks for MMP-9 and normal labial mucosa blocks for MMP-2 were also prepared.
3.3. Histopathological Evaluation
To evaluate the activity of markers, the number of positive cells per 100 cells in five microscopic fields was counted using a light microscope with a magnification of 400 times. Intensity of nucleus and cytoplasm staining was divided into four groups using markers: no expression (score 0), low expression when staining less than 10% (score 1), moderate expression if present in 10 - 50% of cells (score 2) and severe expression when staining present in more than 50% cells: (score 3).
3.4. Statistical Analysis
Data analysis was performed in SPSS 20 software. Kruskal-Wallis test was used to analyze the relationship between variables. P < 0.05 was considered as a significant level in the whole study.
4. Results
4.1. Clinical Characteristics
In this study, 20 patients with oral SCC (53%) and 18 patients with VC (47%) were selected. Among them, 11 males (55%) and 9 females (45%) were in the SCC group, and 8 males (44.46%) and 10 females (55.56%) were in the VC group. The age of patients in SCC and VC groups was between 35 – 77 (48.27 ± 12.11) and 13 – 86 (49.18 ± 19.52) years, respectively.
4.2. Immunohistochemical Findings
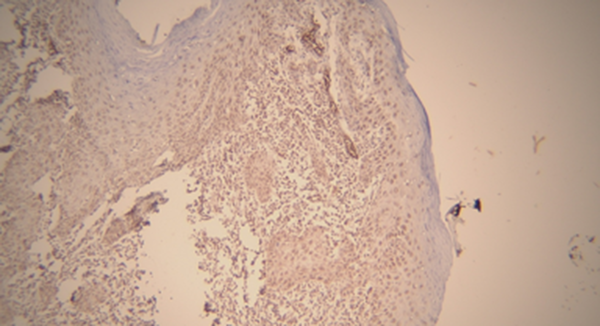
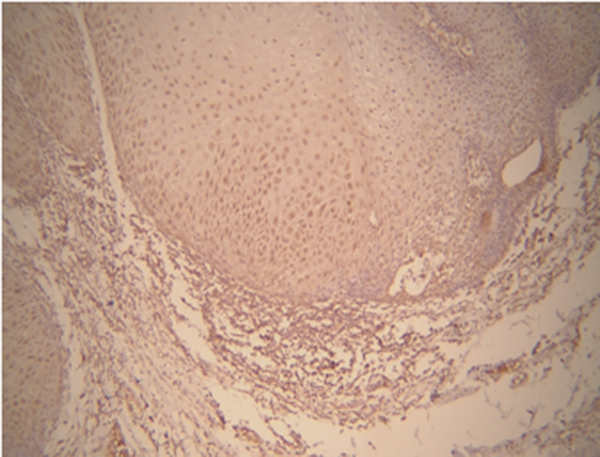
As shown in Table 1, based on Kruskal-Wallis test, there was a significant relationship between MMP-9 and the type of lesion (P = 0.021). Matrix metalloproteinase-9 expression was observed in all oral SCC and VC samples. In addition, the expression level of MMP-9 was very high in most SCC samples, while no signs of very low expression were observed. In contrast, in most VC samples, the expression level was low to moderate. (Figures 1 and 2) Thus, the mean expression level in SCC (groups) was higher than VC groups (Table 1). According to Kruskal-Wallis test, there was a significant relationship between MMP-9 expression level and histopathological grade of tumor in SCC (P = 0.026). In this regard, the mean expression level of MMP-9 was higher in grade III and lesser in lower grades (Table 2).
| Matrix Metalloproteinase-9 Expression Level | Score 0 | Score 1 | Score 2 | Score 3 | Mean ± SD | Median | P-Value |
|---|---|---|---|---|---|---|---|
| Oral squamous cell carcinoma | 0 (0) | 4 (20) | 12 (60) | 4 (20) | 45.60 ± 26.68 | 43.00 | 0.021 |
| Verrucous carcinoma | 0 (0) | 12 (66.67) | 3 (16.67) | 3 (16.67) | 22.39 ± 19.71 | 18.00 |
Comparison Expression Level of MMP-9 in Oral SCC and VC a
| Matrix Metalloproteinase-9 Expression Level | Score 0 | Score 1 | Score 2 | Score 3 | Mean ± SD | Median | P-Value |
|---|---|---|---|---|---|---|---|
| Squamous cell carcinoma grade I | 0 (0) | 3 (15) | 10 (50) | 2 (10) | 32.64 ± 16.40 | 34.5 | 0.026 |
| Squamous cell carcinoma grade II | 0 (0) | 1 (5) | 0 (0) | 1 (5) | 58.67 ± 13.87 | 55.00 | |
| Squamous cell carcinoma grade III | 0 (0) | 0 (0) | 2 (10) | 1 (5) | 93.00 ± 4.58 | 92.00 |
Comparison Between the Expression Level of MMP-9 in Histopathologic Grades of the Lesion a
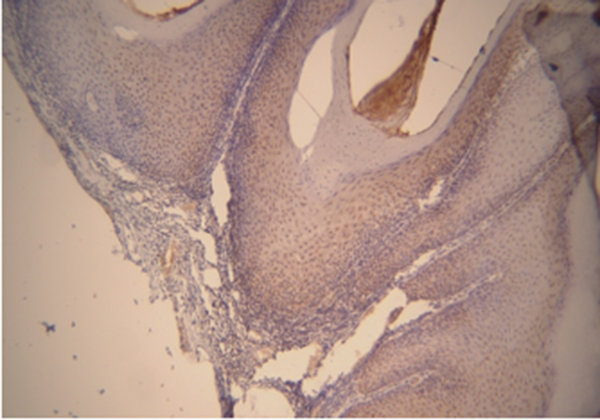
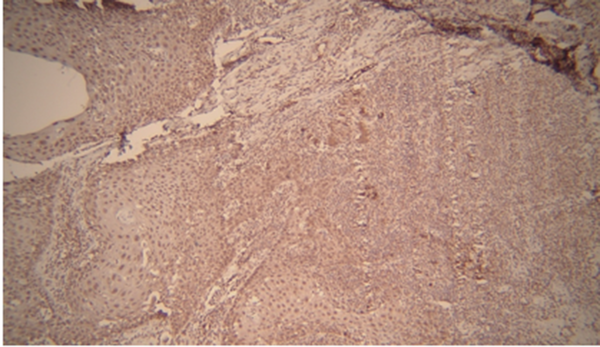
Based on the results of Kruskal-Wallis test, there is a relationship between different grades and levels of expression. However, according to the number and mean scores of each group, SCC grade III and I were more correlated with MMP-2 expression level than the other group. Based on the results of Mann-Whitney test and the mean scores of the two groups, the expression level of MMP-2 in the VC group was higher than the SCC group (Tables 3 and 4) (Figures 3 and 4).
| Matrix Metalloproteinase-2 Expression Level | Score 0 | Score 1 | Score2 | Score3 | Mean ± SD | Median | P-Value |
|---|---|---|---|---|---|---|---|
| Oral squamous cell carcinoma | 0 (0) | 5 (25) | 10 (50) | 5 (25) | 23.50 ± 12.68 | 20.00 | 0.546 |
| Verrucous carcinoma | 0 (0) | 14 (77.77) | 3 (16.66) | 1 (5.55) | 35.28 ± 30.63 | 25.00 |
Comparison Expression Level of MMP-2 in Oral SCC and VC a
| Matrix metalloproteinase-2 Expression Level | Score 0 | Score 1 | Score2 | Score3 | Mean ± SD | Median | P-Value |
|---|---|---|---|---|---|---|---|
| Squamous cell carcinoma grade I | 0 (0) | 3 (15) | 4 (20) | 3 (15) | 25.36 ± 10.09 | 30.00 | 0.551 |
| Squamous cell carcinoma grade II | 0 (0) | 0 (0) | 3 (15) | 0 (0) | 13.33 ± 2.89 | 15.00 | |
| Squamous cell carcinoma grade III | 0 (0) | 3 (15) | 5 (25) | 0 (0) | 25.00 ± 25.98 | 10.00 |
Comparison Between the Expression Level of MMP-2 in Histopathologic Grades of the Lesion a
5. Discussion
This study investigated the expression levels of MMPs 2 and 9 on SCC and VC samples using immunohistochemistry. Gaining more knowledge about these markers will enable earlier diagnosis of high-risk patients, resulting in more accurate monitoring and faster treatment measures. In other words, biomechanical information (in my opinion, immunohistochemical information is correct) is very important to study the progression and spread of tumors (5). In this study, MMP-9 expression was significantly higher in the SCC group, which can be attributed to the more aggressive nature of SCC than VC. In the present study, high expression of MMP-9 was observed in grade III histopathologically in the SCC group, indicating that expression of MMP-9 increases in higher grades. The expression level of MMP-2 in VC was higher than SCC and also varied in different histopathological grades of SCC, so that the highest expression level of MMP-2 was observed in SCC grade I. The MMP expression level of MMP-9 was approximately twice that of MMP-2 in SCC. The results show that MMP-9 is a more reliable marker for diagnosing SCC than MMP-2, and MMP-9 represents the invasive nature of the SCC. However, it can certainly be argued that the expression of MMPs 9 and 2 were positive in all SCC and VC specimens, indicating an increase in the activity of MMPs 9 and 2 in cancerous and precancerous tissues and a role that plays in the progression of the disease. The study also assessed the histopathological grade based on specific criteria: Degree of keratinization, nuclear pleomorphism, invasion pattern, inflammatory infiltration, and morphological parameters (2). The same results were obtained in a series of similar studies. It was observed that there is a significant relationship between histopathological grades and MMP-9 expression, but there is no relationship with MMP-2 (10). Another study showed that MMp-9 can be considered as a reliable marker for measuring metastatic potential in OSCC, and this marker varies according to its grades (4). In a study of MMP-2, the expression level in tumor tissues was significantly higher than in healthy tissues and higher in VC than in SCC, but there was no difference in the expression level of MMP-2 in different SCC grades (11). The most important stage of metastasis is subendothelial basement membrane (BM) degeneration, in which type IV collagen is destroyed at this stage by MMPs 9 and 2. Type IV collagen is a main component of the BM. In other words, the destruction of collagen in the basal lamina leads to the transition from precancerous to malignant conditions (3, 5). Matrix metalloproteinases 2 and 9 can also destroy other BM components such as fibronectin and gelatin (12). Tumor metastasis occurs in several stages, including BM destruction, extracellular matrix regeneration, and angiogenesis (7). Epithelial cells attach to laminin-5, which is a major component of the BM. MMP-2 causes the release and movement of epithelial cells by destroying laminin-5 and preparing them for migration. One of the mechanisms that promotes the migration of tumor cells is the attachment of MMP-2 to the αVβ-3 integrin. This attachment causes the basal structure of the tumor to be reconstructed, so strong expression of MMP-2 is associated with poor survival (12). There is a potential pathological mechanism in tumor cells through which the intercellular adhesion molecule-1 (ICAM-1) forms the binding mechanism at the cellular level for ProMMP-9, leading to ICAM-1 cleavage after activation. This mechanism leads to increased resistance of cancer cells to cytotoxicity mediated by natural killer cells, which increases the survival of migrating tumor cells. This mechanism suggested that MMP-9 plays an important role in tumor cell migration (12, 13). Given that cancer metastasis is the biggest barrier to cancer treatment, the mechanisms by which MMPs can play a role in tumor invasion, and metastasis need to be clearly understood (7). When there is a balance between MMP activity and its inhibitor, there is no pathological effect, but when this balance is lost, a pathological effect appears. Therefore, by inhibiting MMP, a negative effect such as metastasis can be prevented (14). According to a study, the expression of MMPs 2 and 9 was a unique biological characteristic of tumors, indicating their aggressive behavior, especially in the early stages (12). A number of studies have shown that MMPs 9 and 2 are key members of the MMP family that are potentially associated with tumor invasion, progression, and metastasis (15, 16). Infact, a greater increase in the activity of MMPs 2 and 9 was observed in malignant tissue than in adjacent soft tissue (5). In addition, there was a significant relationship between increased expression levels of MMPs 9 and 2 and invasion of esophageal cancer vessels with metastasis compared with esophageal carcinoma without metastasis (5). This has been widely observed in epithelial dysplasia, suggesting that MMP-9 may play an active role in the transition from dysplasia to carcinoma (1). It is important to accurately determine the expression level of MMP-9, which plays an important role in tumor invasion and metastasis, as it can aid the development of a drug that can inhibit MMP-9 (2). Another study showed that activated NFkB leads to activation of MMPs 2 and 9 and therefore inhibition of NFkB can leads to decreased expression of MMP-9 (12). Indeed, determining the role of MMPs 9 and 2 in cancer progression is important because it allows researchers to develop inhibitory MMPs 2 and 9, which completely alter collagen structure or inhibit collagen MMP binding. Recent findings suggest that the development of selective MMP inhibitors is more effective (17). There are also different opinions on the importance and role of MMPs 2 and 9 in tumor invasion. For example, in some studies, the expression of MMPs 2 and 9 have not been considered as a reliable function in tumor invasion (9), but in others, they are acceptable indicators (18, 19). Some studies have reported no association between MMP-9 expression and initial tumor size and metastasis (20, 21). However, a study linked high expression levels of MMP-9 in oral SCC to local involvement or remote metastasis (22). Many studies have shown that MMPs 2 and 9 are significantly expressed in head and neck tumors, leading to a greater chance of cancer progression and invasion (2, 22-24). Poor prognosis and tumor progression are due to failure of physical barriers and regulation of cell proliferation; angiogenesis is involved in all stages of tumor development. Matrix metalloproteinase-9 expression leads to further tumor growth and initial metastasis through activation of growth factor and its expression in the matrix (12). Certain mechanisms control MMP through hereditary gene polymorphism, which can regulate MMP activity through DNA methylation and post-transcriptional mechanisms, but further studies are needed to better understand the issue (7). Matrix metalloproteinases can lead to many pathological activities, one of which is malignant tumor metastasis. Therefore, by controlling the activity of the MMPs, big steps are taken to overcome cancer.
5.1. Conclusions
The results of this study on the expression of MMP-9 in VC and SCC showed this marker was positive for all SCC results, with moderate to severe expression levels. Matrix metalloproteinase-9 was positive in most VC samples with low to moderate expression levels in most cases. The mean expression level of MMP-9 in SCC was higher than in VC. According to this study, the expression level of this marker varies in different histopathological grades of SCC. Matrix metalloproteinase-2 was positive in all cases of SCC and VC, and its expression level was higher in VC. The mean expression level of MMP-2 in VC was also higher than SCC. The mean expression level of MMP-2 in SCC grade I was higher than other groups. As a result, it can be used as a reliable marker in evaluating disease progression. Future studies are proposed to investigate the mechanisms of gene expression and MMP inhibitors.