1. Background
Spinal anesthesia (SA) is a frequently used method by anesthesiologists in patients who may not need general anesthesia or have a contraindication for applying general anesthesia. Nevertheless, numerous contemplations need to be considered by anesthesiologists when using this method. The most critical issue for the proper management of SA is the management of anesthesia according to the duration of surgery, which is especially important in addicted people. Significant shortening of SA duration in opium-addicted patients, lower level of sensory block in these individuals, and faster return of motor block are also specific contemplations in such population (1-3). Alongside inducing the block with required anesthetic agents such as Bupivacaine, researchers have tried to find some extra interventions to manage such contemplations.
The relatively short duration of the effect of local anesthetics has been addressed by encapsulation in drug delivery systems. It is highly believed that concurrent administration with a single compound that produces an adjuvant effect on nerve block but without intrinsic local anesthetic properties can further prolong the nerve block effect (4). Co-administration of dexmedetomidine or dexamethasone are among the interventions that can enhance the effect of local anesthetic agents when used as an adjunct during peripheral nerve block (5). However, when it comes to SA, although intravenous (IV) and intrathecal administration of dexmedetomidine or dexamethasone, alongside the subarachnoid injection of an anesthetic agent, has been frequently examined (6-9), to the best of our knowledge, these two drugs have never been compared with each other in this regard. Also, although the effects of various IV drugs during SA on postoperative opium use have been shown in various studies, the effects of these drugs on the level of spinal anesthesia and motor block have not been studied so far. Noteworthy, most trials have excluded (or not separated) opium-addicted patients and assessed the effects of interventions just in non-opium addicts or overall. Apparently, many patients may be opium-addicted, and we believe that special trials in this population would be valuable.
2. Objectives
We decided to conduct a randomized clinical trial (RCT) to compare the effect of IV administration of dexamethasone and dexmedetomidine alongside the subarachnoid injection of bupivacaine in terms of spinal anesthesia quality in opium-addicted patients.
3. Patients and Methods
3.1. Trial Design
This parallel RCT was conducted in Imam Hosein Hospital, Tehran, Iran, following the Declaration of Helsinki principles in experimental studies. The Ethics Committee of Shahid Beheshti University of Medical Sciences approved the study proposal (IR.SBMU.MSP.REC.1400.202). The study protocol was registered on www.irct.ir on 2021-12-01, and the code IRCT20120430009593N15 was assigned. After that, sampling was performed from 2021-12-21 until 2022-12-20. Patients were enrolled just in case of receiving their signed informed consent.
3.2. Participants
We included opium-addicted patients (defined as those who regularly used opium within the previous six consecutive months) in the age range of 18 to 65 years with the American Society of Anesthesiologists (ASA) class of I/II candidates for surgery under SA. Pregnancy, body mass index (BMI) > 30, coagulation disorders, multiple sclerosis, local infection at the site of the lumbar region, known allergy to local anesthetics, sepsis, diabetic neuropathy, and bradycardia (heart rate less than 50 beats/min) were considered as the exclusion criteria. Patients’ demographic and baseline characteristics, including age, sex, type of abused drug, duration of opioid abuse, anatomical surgery location, and duration of surgery, were all registered in a prepared checklist.
3.3. Sample Size
Considering α = 0.05 and β = 0, and using the below formula, the least required sample size in each group was calculated as 27 cases.
3.4. Randomization and Allocation
We used block stratified randomization software window version 6.0 to create a random number table. The study population was divided into two groups of 30 patients. At the beginning of each person’s entry, his/her number was matched with the table, so the type of group, dexmedetomidine or dexamethasone, was specified.
3.5. Intervention
In one group (A), dexmedetomidine (vials of 2 cc/200 μg; Exir Drug company) at a dose of 0.5 μg/kg body weight was injected intravenously 10 minutes before surgery and then at a dose of 0.5 μg/kg body weight during surgery. In another group (B), 8 mg dexamethasone (ampules of 2 cc/8 mg; Alborz Drug Company) was injected intravenously 10 minutes before surgery, and then normal saline at a rate of 0.5 μg/kg/h was infused during surgery. Patients in both groups were simultaneously hydrated with 500 cc of normal saline, and then spinal anesthesia was performed with 3.5 cc of Bupivacaine 0.5% in the lateral position. The pinprick test assessed the sensory level at the T10 level, and the Bromage scale evaluated the onset of motor block.
The patients were evaluated for variables related to patient hemodynamics (including blood pressure and heart rate, nausea or vomiting, and drowsiness), and spinal anesthesia quality (by examining the level of sensory block and motor block) was evaluated every three minutes up to 15 minutes, every 5 minutes up to 30 minutes, and every 15 minutes up to two hours. Evaluation of spinal anesthesia quality began three minutes after spinal anesthesia based on a pinprick test for the sensory block and a Bromage scale test for the motor block. The onset of sensory block was considered when the patient did not feel the needle in the T10 dermatome. Motor evaluation is done based on the Bromage scale so that in the mentioned intervals, the patient’s movement scores were evaluated and recorded, and score one was considered the time of starting the movement block.
During the surgery, the patients had the same fluid therapy and received no other analgesic or hypnotic agent. The patient’s hemodynamics were continuously monitored to assess the need for extra management. In the case of hypotension (defined as a decrease in mean arterial pressure (MAP) of at least 20% of baseline), 10 mg of ephedrine was injected. Also, in the case of bradycardia (defined as HR < 50), the patient was treated with 0.5 mg of IV atropine.
3.6. Outcomes
The primary outcomes of this trial were the onset of sensory block, the onset of motor block, the regression of two levels of sensory block, and the duration of motor block. Total analgesia time and time to the first analgesia requirement were the secondary outcomes of this study. Total time of analgesia means the total time when the patient had no pain at the surgical site, i.e., from the time of spinal anesthesia to the onset of pain at the surgical site with pinprick stimulation. The first time to request analgesia was achieved when the patient experienced pain. In this case, the pain score was checked. If the NRS was more than 3, 5 mg of morphine was prescribed.
3.7. Blinding
The drugs were given by one anesthesiologist who knew the groups. Another anesthesiologist who was unaware of the groups performed SA and assessed the outcomes. The patients were also blinded to the groups.
3.8. Statistics
This study used a t-test to compare the two groups’ main variables (motor block). Chi-square non-parametric test was used to compare the baseline variables. All comparisons and statistical analyses were performed in SPSS 26 software, and P < 0.05 was considered a significance level.
4. Results
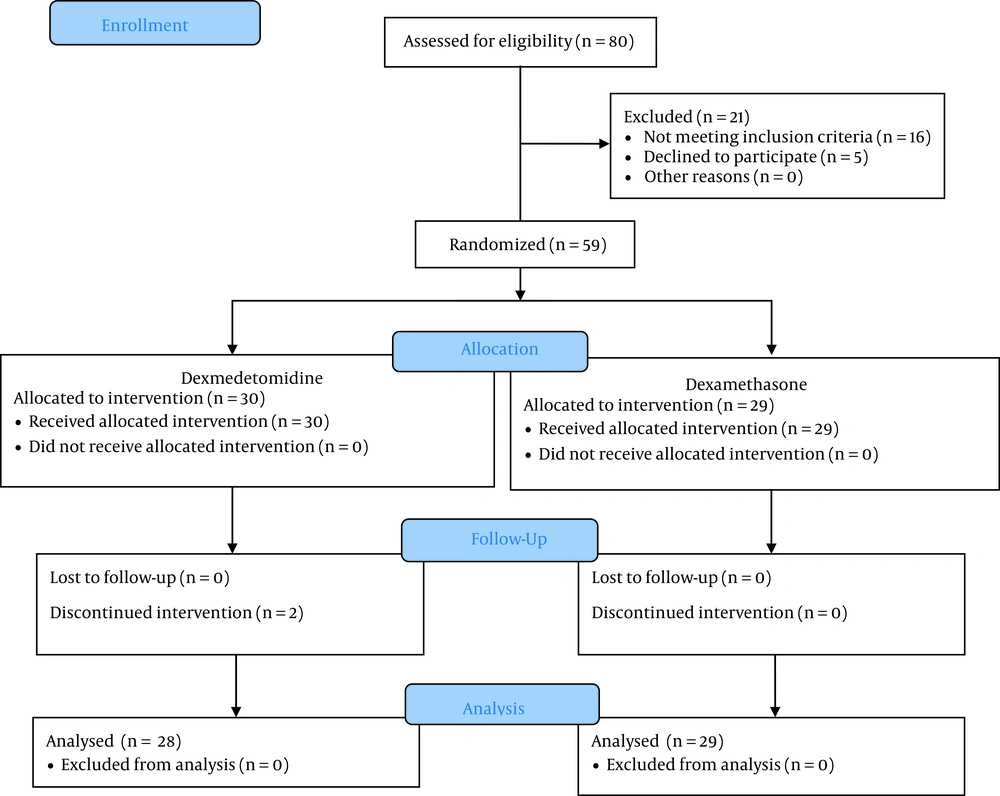
Totally, 57 patients divided into two groups were included in the final analysis. The CONSORT flowchart of the study is presented in Figure 1. The mean age of the patients was 42.34 ± 14.34 (range: 19 - 63) years and 41.82 ± 12.45 (range: 25 - 63) years in the dexamethasone and dexmedetomidine groups, respectively (P = 0.341). The mean duration of surgery in the dexamethasone and dexmedetomidine groups was 80.34 ± 40.04 (range: 25 - 180) minutes and 92.14 ± 35.76 (range: 30 - 135) minutes, respectively (P = 0.719). Details of baseline characteristics of the study patients are presented in Table 1. The results showed that the two groups differed significantly in none of the assessed baseline variables.
| Variable | Dexmedetomidine (n = 28) | Dexamethasone (n = 29) |
|---|---|---|
| Sex | ||
| Male | 27 (96.4) | 28 (96.6) |
| Female | 1 (3.6) | 1 (3.4) |
| P-value | < 0.001 | < 0.001 |
| Type of abused drug | ||
| Opioid | 23 (82.1) | 19 (65.5) |
| Opioid + methamphetamine | 2 (7.1) | 2 (6.9) |
| Methadone | 3 (10.7) | 7 (24.1) |
| Opioid + Methadone | 0 (0.0) | 1 (3.4) |
| P-value | < 0.001 | < 0.001 |
| Duration of opioid abuse | ||
| < 6 months | 3 (10.7) | 1 (3.4) |
| ≥ 6 months | 25 (89.3) | 28 (96.6) |
| P-value | < 0.001 | < 0.001 |
| Surgery location | ||
| Foot | 1 (3.6) | 1 (3.4) |
| Femur | 4 (14.3) | 2 (6.9) |
| Knee | 5 (17.9) | 4 (13.8) |
| leg | 10 (35.7) | 11 (37.9) |
| Lower abdomen | 0 (0.0) | 4 (13.8) |
| Pelvic | 5 (17.9) | 5 (17.2) |
| Ankle | 3 (10.7) | 2 (6.9) |
| P-value | 0.084 | 0.013 |
a Values are expressed as No. (%).
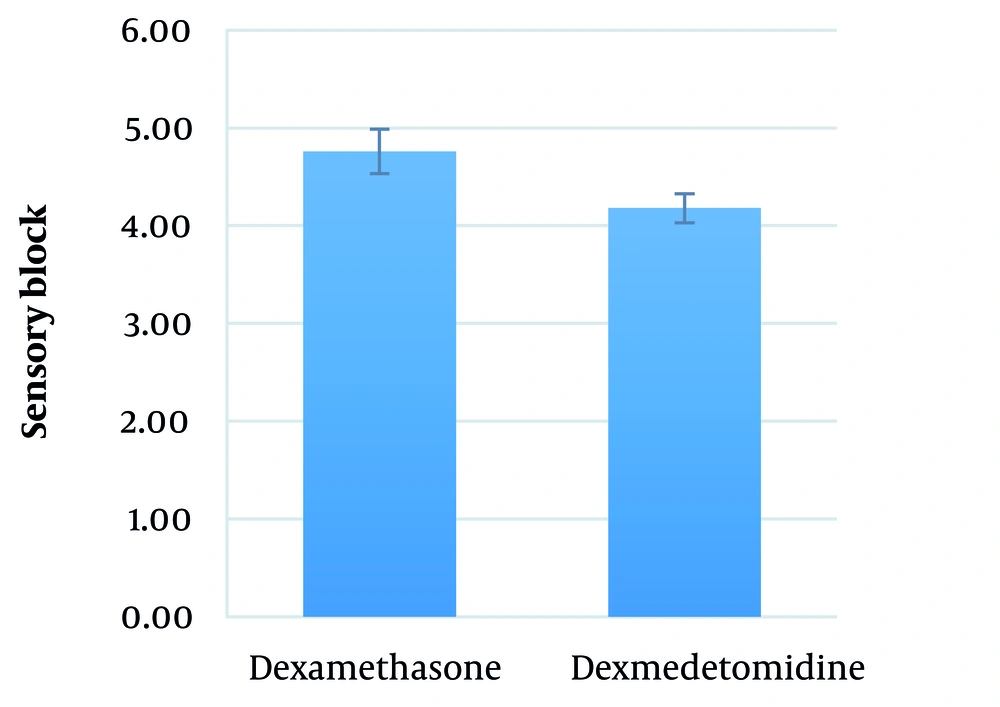
The mean duration between performing SA and the onset of sensory block in the dexamethasone and dexmedetomidine groups was 4.8 ± 2.2 (range: 3 - 9) minutes and 4.2 ± 1.9 (range: 3 - 9) minutes, respectively (P = 0.290) (Figure 2).
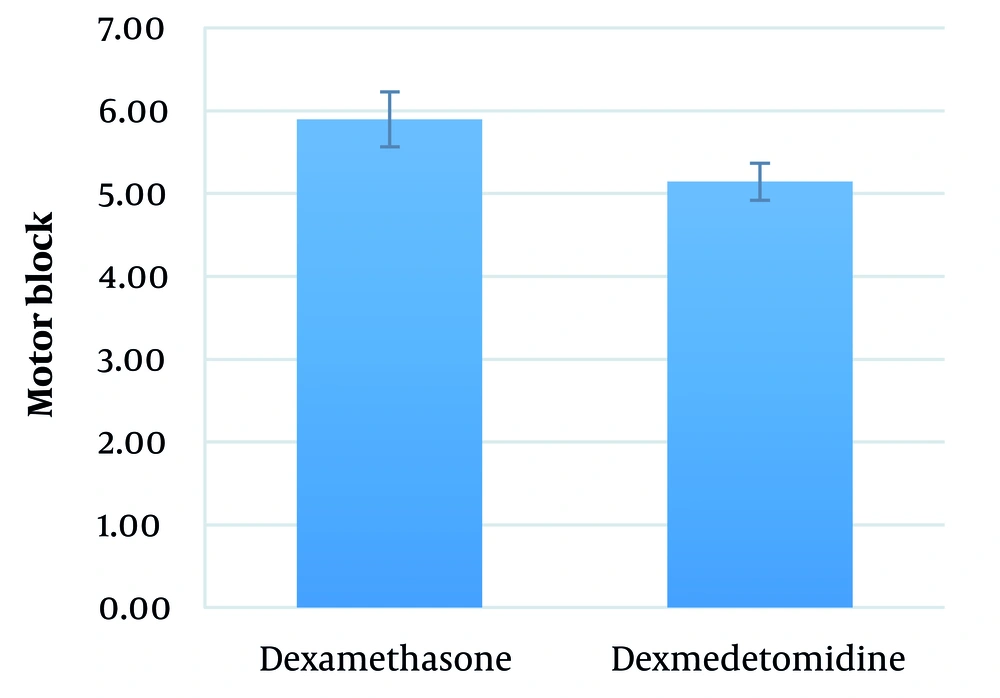
The mean duration between performing SA and the onset of motor block in the dexamethasone and dexmedetomidine groups was 5.9 ± 2.6 (range: 3 - 12) minutes and 5.1 ± 2.3 (range: 3 - 9) minutes, respectively (P = 0.251) (Figure 3).
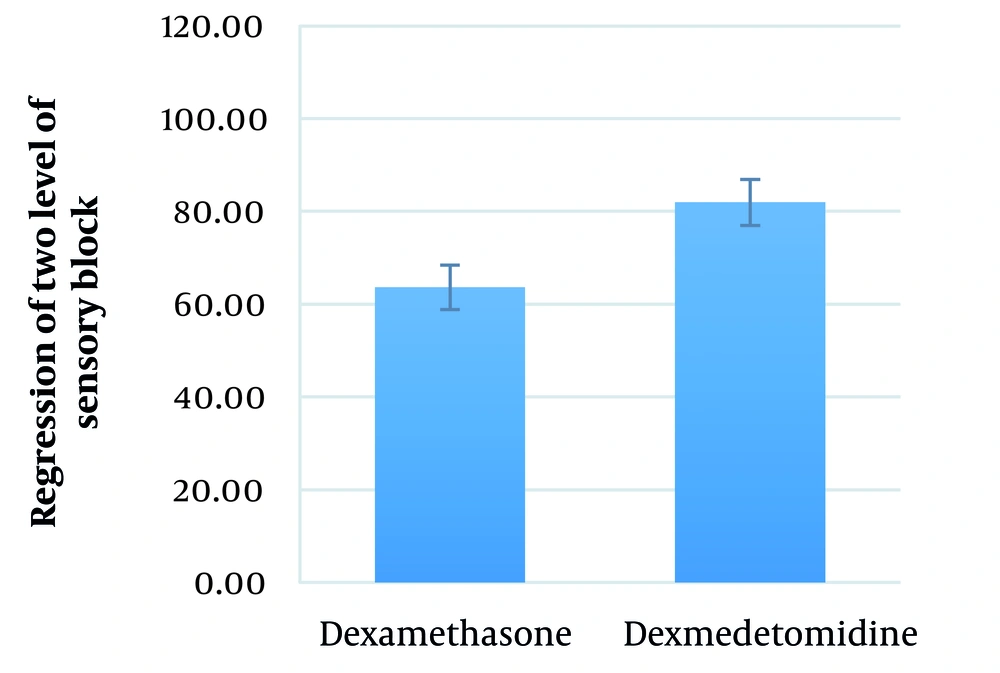
The mean duration between performing SA and the regression of two levels of sensory block in the dexamethasone and dexmedetomidine groups was 63.6 ± 27.7 (range: 30 - 120) minutes and 82.0 ± 17.1 (range: 45 - 105) minutes, respectively (P = 0.004) (Figure 4).
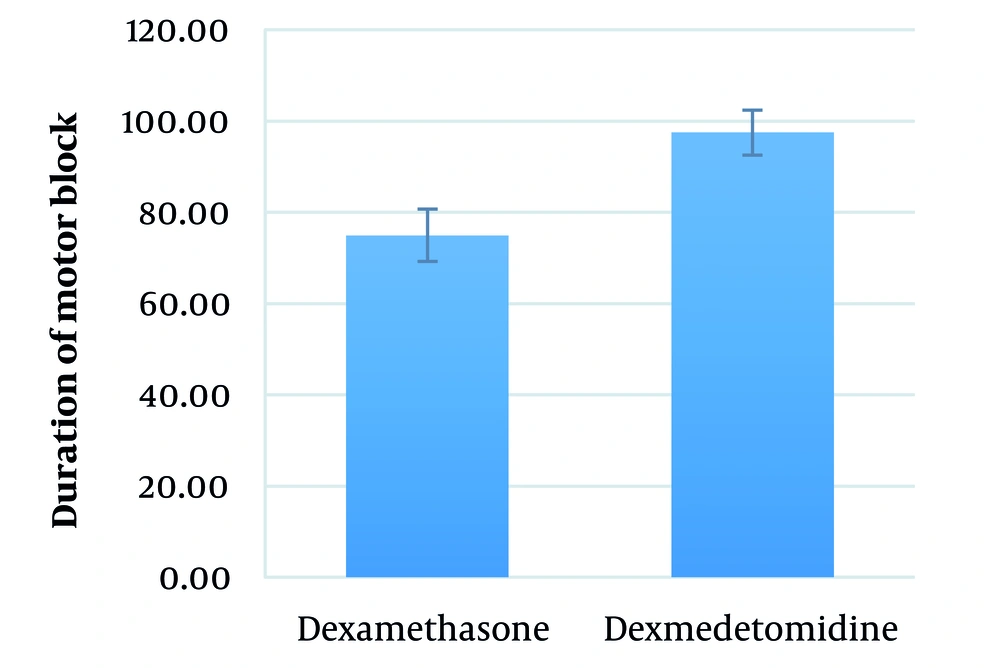
The mean duration of motor block in the dexamethasone and dexmedetomidine groups was 75.0 ± 32.1 (range: 30 - 150) minutes and 97.5 ± 19.4 (range: 60 - 120) minutes, respectively (P = 0.377) (Figure 5).
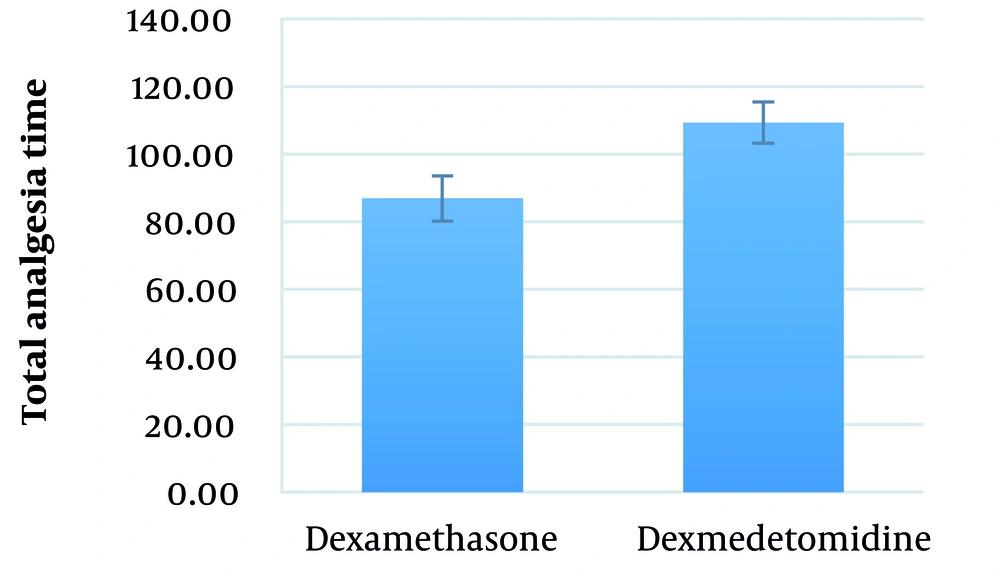
The mean total analgesia time in the dexamethasone and dexmedetomidine groups was 86.9 ± 32.9 (range: 45 - 180) minutes and 109.3 ± 16.3 (range: 75 - 135) minutes, respectively (P = 0.002) (Figure 6).
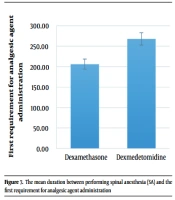
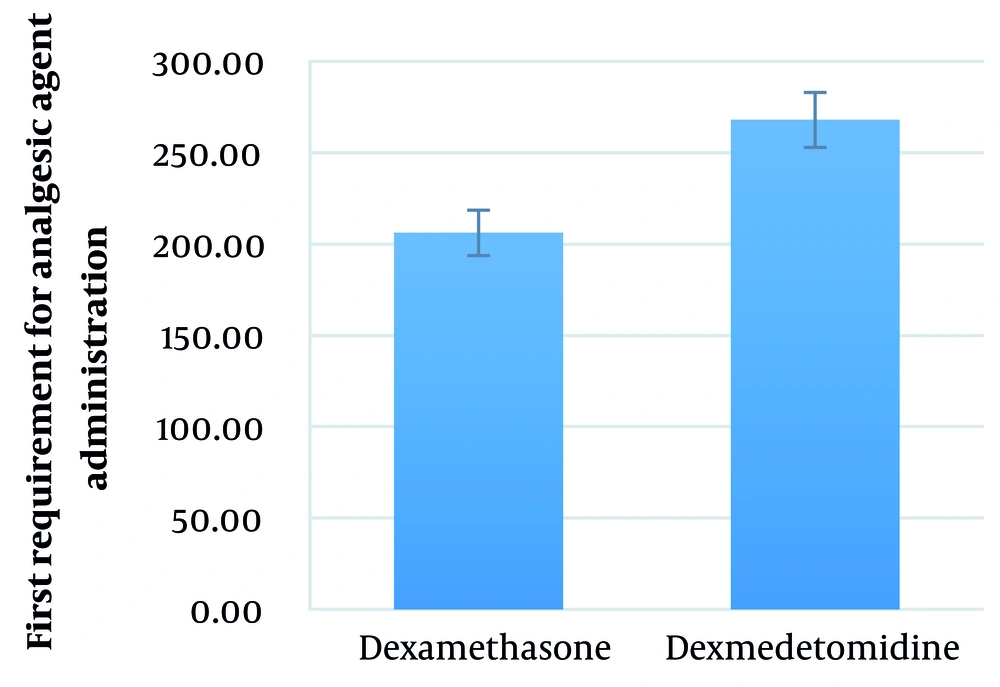
The mean duration between performing SA and the first requirement for analgesic agent administration in the dexamethasone and dexmedetomidine groups was 206.21 ± 93.19 (range: 55 - 420) minutes and 267.86 ± 76.02 (range: 105 - 465) minutes, respectively (P = 0.008) (Figure 7).
5. Discussion
The mean duration between performing SA and the regression of two levels of sensory block, the mean total analgesia time, and the mean duration between performing SA and the first requirement for analgesic agent administration was significantly shorter in the dexamethasone group than in the dexmedetomidine group. The mean duration between performing SA and the onset of sensory block and the mean duration between performing SA and the onset of motor block was longer in the dexamethasone group than in the dexmedetomidine group. The mean duration of motor block was shorter in the dexamethasone group than in the dexmedetomidine group, but these differences were not statistically significant. Such evidence was not previously available in the statistical population of opium-addicted patients, and this study would pave the way for further studies. As we mentioned before, significantly shorter duration of SA, lower level of sensory block, and faster return of motor block in opium-addicted patients cause problems while performing SA in this population. It seems that IV administration of dexmedetomidine concurrent with SA could provide a more comfortable space for surgeons and anesthesiologists.
A systematic review evaluated the effect of IV administration of dexmedetomidine concurrent with SA. The authors concluded that compared with placebo, the intervention could prolong the duration of sensory block, motor block, and time to first analgesic request (6). Of course, knowing the results of this study, we designed ours and tried to take a step forward.
A study compared the effects of dexamethasone and dexmedetomidine co-injection added to ropivacaine on the onset and duration of axillary plexus nerve blocks. Both drugs were equally effective in extending the duration of anesthesia, but neither drug significantly affected the onset of anesthesia (10). While we found that the mean total analgesia time was more in the dexmedetomidine group than in the dexamethasone group, we did not see any difference in the onset of anesthesia between the two groups. Dexamethasone was superior to dexmedetomidine in two systematic reviews conducted to determine the superiority of these two drugs as a perineural adjunct for supraclavicular brachial plexus block. Compared to dexmedetomidine, dexamethasone prolonged the duration of analgesia without prolonging sensory/motor blockade.
In comparison, dexmedetomidine increased rates of hypotension and sedation (5, 11). Meanwhile, another systematic review concluded that dexamethasone and dexmedetomidine had equivalent analgesic effects in peripheral nerve blocks (12). We did not compare the rate and level of sedation in the two groups, but when it came to vital signs, there were no differences between the two groups in our study. However, if we want to assess the total superiority in our trial, we vote for dexmedetomidine. However, different interventions performed in our study and other previous studies in this area make it difficult to compare and analyze the results, which warrants additional high-quality RCTs in the future to provide more robust evidence.
What we already knew based on available evidence was that IV dexmedetomidine, compared with placebo, could prolong the duration of anesthesia and decrease the need for opioid use after recovery (9, 13-15). On the other hand, the addition of intrathecal dexamethasone to anesthetic agents significantly improved the duration of sensory block in SA (8, 16-18), and also available evidence shows that the addition of IV dexamethasone to anesthetic agents improved the quality of SA in previous studies (19-21). Nevertheless, to the best of our knowledge, IV dexmedetomidine has never been compared with IV dexamethasone in this regard. Our attempt to find a study that compared the two drugs somehow led to finding one RCT in the literature, in which dexamethasone and dexmedetomidine were compared as an adjuvant to intra-articular bupivacaine in arthroscopic surgeries. It should be mentioned that all patients received both intra-articular injections and SA with Bupivacaine. The patients were divided into three groups that either received dexamethasone, dexmedetomidine, or normal saline as an adjuvant for intra-articular bupivacaine. Also, SA was performed with the same technique and drug for all patients. They found no differences between the three groups in terms of the onset of sensory block, the onset of motor block, the regression of two levels of sensory block, and the duration of motor block. However, the time to the first analgesic requirement was significantly shorter in the normal saline group than in the other two groups; there was no difference between dexamethasone and dexmedetomidine groups in this regard (22). We think the different intervention processes in this study and what we did can explain the different findings.
5.1. Suggestions for Future Research
Injection of bupivacaine combined with both dexamethasone and dexmedetomidine as local anesthetics prolonged the duration of anesthesia to three folds compared with the injection of bupivacaine alone when this combination was used in terms of nerve block in an animal study (4). Another study with the same hypothesis compared the combination of dexamethasone and dexmedetomidine as adjuvants to bupivacaine versus using each agent alone in the pediatric caudal block. The authors concluded that the addition of combined dexmedetomidine and dexamethasone to bupivacaine would be more suitable than each drug if used alone (23). Reviewing such evidence and thinking about our findings in the current study have made us design another study to compare the efficacy of IV administration of combined dexmedetomidine and dexamethasone versus each of these two agents alone concurrent with SA in the near future.
5.2. Limitations
We believe that some other variables remained to be assessed during such trials; for example, we did not compare the rate and level of sedation in the two groups.
5.3. Conclusions
It seems that the quality of spinal anesthesia in opium-addicted patients who received concurrent IV dexmedetomidine was better than that of those who received concurrent IV dexamethasone, as we found that opium-addicted patients who received concurrent IV dexmedetomidine showed more extended duration between performing SA and the regression of two levels of sensory block, total analgesia time, and duration between performing SA and the first requirement for analgesic agent administration than who received concurrent IV dexamethasone.