1. Background
It is estimated that about 12.7 million people inject drugs globally, and 1.7 million (13%) of those are living with HIV (1). The significant spread of HIV infection amongst people who inject drugs (PWID) has been shown in many countries such as Europe, Asia and North America (2, 3). In Iran, there are approximately 170,000 - 230,000 PWID of whom, 15% are infected with HIV (4, 5). Over two-thirds of all new identified HIV cases have been attributed to unsafe injection (4, 6-8). To reduce the risk and harms associated with injections, needle and syringe programs (NSP) have been developed and implemented widely (9-12). In Iran, NSP is the cornerstone of HIV prevention interventions and has been implemented in different community settings since 2002 (8, 10, 13-15). NSP is being delivered through drop-in centers, (DIC) for those who have access to services and by outreach and second-hand exchanges (7, 8). The DIC is a place that addresses the health needs of PWID. Additionally, it supports the rights of people using drugs for treatment and care services that are respectful and non-discriminatory. The DIC also functions as a fixed outlet for a needle and syringe exchange program (NSEP), where in these programs they can exchange their used needles for new ones at the DIC. These centers and outreach needle and syringe programs provide sterile needle and syringe services, deliver training on safe injecting practices on overdose prevention as well as providing condom and safe sex education (16). The effectiveness of these programs have been studied and reported to be an effective intervention in developed countries (9, 17-20), however the effect of NSP with different coverage was not studied in Iran. The number of needles and syringes given to a person who injects drugs should be corresponded with his or her frequency of injection; otherwise, the effect of this program may be compromised or even be harmful (21). In this study, our hypothesis states that low NSP coverage (insufficient proportion of all drug injections becoming safe through distributed syringes) will not effectively reduce the harms associated with the injection and so associated with high transmission of HIV amongst PWIDs. Using empirical data collected from two drop-in centers and an outreach team in Kermanshah, Iran as well as a mathematical model, we assessed the impact of insufficient NSP on HIV incidences among PWID.
2. Objectives
The aim of this study was to determine the effectiveness of NSP coverage in prevention of HIV incidence among PWIDs.
3. Patients and Methods
3.1. Study Design
This was a model study to predict the annual incident of HIV amongst PWID with sufficient and insufficient client-level coverage of NSPs. To explore the expected national annual incidence associated with different levels of sterile syringe distribution we used a mathematical model, which was designed by Wilson and colleagues (21). Sufficient needles and syringes defined to potentially have one clean needle/syringe available per injection and were estimated from frequency of injection and syringes pick up. The input data used for our mathematical model was collected by a self-reported interview-based questionnaire completed by 470 PWIDs in Kermanshah, Iran. Study participants were recruited from the community and NSP sites between September and December 2014. PWID from NSP sites were recruited at the facility by convenience sampling; where as community-based people were recruited by outreach, random street-walk and peer-referral (snowball using referral coupons) sampling. People who met the eligibility criteria were approached and asked to participate in our study after providing informed consent. The criteria to become eligible and be recruited into the study was that the male clients were 18 years of age or older, reported drug injection within the last month prior to the interview, and gave written consented to participate in the study. The study protocol and all the procedures were reviewed and approved by the ethical committee of the Kerman University of Medical Sciences (ethical code k/93/204). At the DIC, a trained male psychologist introduced the study objectives, explained the risk and benefits of participation in the study as well as assessing the eligibility criteria. Next, the psychologist interviewed each consented individual using a standardized structured questionnaire based on the client-level coverage. Participants were then divided into two groups: those with the coverage less than 100% (insufficient coverage) and those with the coverage of 100% or more (sufficient coverage). We used a deterministic model to explore the expected annual occurrence of HIV associated with the different coverages of NSP. The expected number of new HIV infections (I) among people who inject drugs was estimated by the Equation 1:

N: number of PWID in each group; n: number of injections per year for each PWID; S: proportion of IDUs who share syringes; q: proportion of their injections may shared; r: infected people in a sharing group; p: initial prevalence HIV among PWID; ρ: proportion that cleaning syringe occurs before sharing injections; δ: average number of times each shared syringe is used before disposal; β: HIV transmission probability per injection with contaminated syringe; ε: effectiveness of syringe cleaning.
3.2. Model’s Parameters
We parameterized the model using behavioral and epidemiological data collected in an empirical study of PWID. Using the empirical data, in those PWID with and without sufficient NSP coverage, we estimated the proportion of people who inject drugs may share their syringes (p); proportion of their injections they may share (q), average size sharing groups (m) and HIV prevalence amongst PWID (P). Other biological parameters such as risk of HIV transmission per injection with a shared injection (β), and effectiveness of syringe cleaning to prevent virus transmission (ε) were obtained from literature (22-30). A summary of such parameters for each group, with and without sufficient NSP coverage, is presented in Table 1.
| Input Parameters (PWID Reported Behavior) | Source of Data | Probability Distribution (Model Inputs) | Model Inputs for | |
|---|---|---|---|---|
| Access Insufficient (n = 250) | Access Sufficient (n = 220) | |||
| Average size of a sharing group in each party | Estimated from Kermanshah Survey (9) | Triangular (2 - 3.4) | 2.33 | 2.21 |
| HIV transmission probability per injection with contaminated syringe | Published literature (22-24) | Triangular (0.003 - 0.01) | 0.007 | 0.007 |
| Effectiveness of syringe cleaning to prevent virus transmission | Published literature (25, 26, 30) | Triangular (0.4 - 0.6) | 50% | 50% |
| Average number of injections per PWID per year | Estimated from Kermanshah Survey (9) | Triangular (650 - 2200 | 1180 | 1080 |
| Proportion of PWID who share syringes | Estimated from Kermanshah Survey (9) | Triangular (0.1 - 0.2) | 18% | 10% |
| Proportion of injections that are shared by PWID per year | Estimated from Kermanshah Survey (9) | Triangular (0.01 - 0.5) | 0.1% | 0.06 |
| Average no. of times each shared syringe is used before disposal per year | Estimated from Kermanshah Survey (9) | Triangular (1- 4) | 2.66 | 2.12 |
| Initial prevalence of HIV among PWID | Estimated from Kermanshah Survey (9) | Beta (0.1 - 0.25) | 15% | 15% |
| Proportion of syringes cleaned before reusing | Estimated from Kermanshah Survey (9) | Triangular (0.2 - 0.9) | 47% | 60% |
Model Input Parameters and Distribution Types Used in Uncertainty Analysis in two Groups
3.3. Estimation of HIV Averted
To capture the uncertainty in the model parameters, we applied a Monte Carlo simulation model. This was to assess how the uncertainty in the input parameters affects the overall impact estimates. To do this, for each model input we developed a probability distribution of the potential range of values that it could take (Table 1). We used the distribution of data we observed in the empirical study as the uncertainty measure. For others parameters, we used the literature to assign the proper distribution. Then, we ran the Monte Carlo simulation with 10,000 draws in two scenarios, first using the parameters for PWID with sufficient NSP coverage and then for PWID without sufficient NSP coverage. The estimated HIV annual incidence was reported as the main output of the model with a simulation interval (SI 95%). We considered the relative risk reduction (RRR) of HIV incidences (HIV averted) as the NSP effectiveness measure. Furthermore, we conducted a probabilistic one-way sensitivity analysis on every single parameter to assess its effect on HIV occurrences. The effect was reported as tornado plot. We used the Ersatz software (http://www.epigear.com; Monte Carlo add-in program for MS Excel) for modeling and calculation.
4. Result
4.1. Characteristics of Study Participants in Kermanshah Survey
A total of 470 men who injected drugs participated in the study; the mean age ± SD was 33.8 ± 8.58 years. The majority of participants were unemployed (64%), had a monthly income less than $150 (85%) and homeless (42%). Concerning their HIV status, 16 % did not know their sero-status and 15% of them were reported as positive. The mean for first time drug usage was 21.2 ± 11.5 years of age and the mean for first time drug injections was 26.7 ± 12.5 years of age.
4.2. Injection-Related High-Risk Behaviours
As reported in Table 1, subjects reported a higher average number of injection per week (21.28 ± 15.11 vs. 7.74 ± 7.58), sharing injections per week (3.10 ± 5.42 vs. 0.4 ± 0.97) and shared person in each party (2.33 ± 2.0 vs. 2.37 ± 0.80) in insufficient group, compared to sufficient group, all differences were statistically significant (P < 0.001). Regarding the cleaning of the syringe before it is reused, 47% of subjects with low coverage and 60% of subjects with sufficient coverage reported cleaning the syringe before reusing.
4.3. Model Estimates of Impact (HIV Averted)
Overall, we estimated the number of new HIV infections due to sharing injections with insufficient NSP coverage as 40.4 per 1000 PWID. Amongst those with sufficient NSP coverage, the HIV infection rate was estimated as 10.2 per 1000 IDU. The impact projections are shown in Table 2. The results of the uncertainty analysis showed that NSP with sufficient coverage could avert 30 (95%SI: 15.1-36.2) new HIV infections per 1000 IDUs per year. In others words, HIV infections prevented by sufficient coverage among PWID is about 75.2% (95%SI: 70.4%–78.1%).
4.4. Sensitivity Analysis
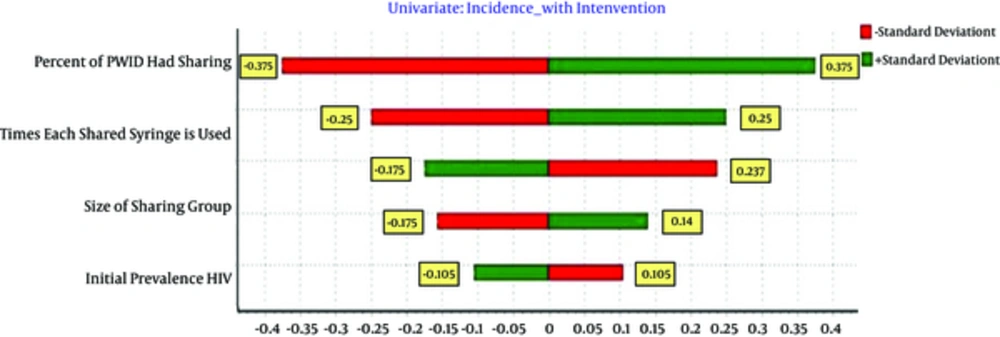
For sensitivity analysis, each input parameter is changed ± 1SD, and its effect on effectiveness is calculated and shown by Tornado Plots (Figure 1). This figure represents the possible HIV averted range given the variability around each parameter. Sensitivity analysis by constructing tornado plots demonstrate the robustness of the model in which changing the input parameters ± 1SD results in less than 0.5 SD in outputs (HIV Averted). The average number of times each shared syringe is used before disposal, average size sharing groups, percent of PWID had sharin and prevalence of HIV amongst PWID were the most behavioral factors associated with NSP effectiveness (Table 2).
| Outcome Measure | Insufficient Coverage Mean (CI 95%) | Sufficient Coverage Mean (CI 95%) | Difference |
|---|---|---|---|
| HIV incidence | 40.4 (28.2 - 56.1) | 10.2 (3.3 - 16.4) | 30.2 (15.1 - 36.2) |
Uncertainty Analysis for Cumulative Incidence of HIV Infection per 1000 PWID per Year Sufficient/Insufficient Coverage
5. Discussion
The analysis used economic analysis and mathematical modeling to estimate the effectiveness of an NSP intervention in Kermanshah, Iran. The results suggest that, despite the high HIV prevalence, NSP’s with sufficient coverage can be effectively averted to about 30 new cases of HIV a year per 1000 active PWID, with an even more effect when including their sexual partners. Needle sharing, which is still common amongst injecting drug users (18%) who are linked to NSP services can be reduced more to 11%, if they have been provided sufficient number of needles and syringes though ongoing NSP activities. Many studies have reported that NSP or NSEP can decrease syringe sharing or HIV transmission but few studies have used mathematical modeling to consider the impact of coverage of NSP on HIV occurrences (31). In present studies, for the first time after implementing and scaling up the NSP in Iran, we used mathematical models in order to estimate HIV infections averted by ensuring sufficient coverage of NSP. This model can also be used to estimate how the expected incidence may change due to changes in syringe distribution through NSPs (30). Worldwide, many studies have reported that NSP or NSEP can decrease HIV incidence or HIV transmission (6, 30, 32-35). Vickerman et al. modeled the impact NSP and showed that by increasing the coverage of NSP, HIV incidences will decrease to 47% (30). Similar to other studies, we also found that injecting drug users are connected with two to three people who also inject drugs (36). Being provided sufficient syringes and needles ensure that they have enough sterile needles/syringes for their injection; and such connections amongst PWID to be remained safe and reduce the risk of needle sharing and further transmission of infection amongst them. However, needle sharing may occur for social reasons, and not always due to lack of sterile syringes available to them (30). Our study cannot differentiate such effects and so the true effect for increasing the coverage of NSP could be less than what we observed. As previously stated, another limitation to our study is ignoring the indirect effect of increasing the coverage of NSP amongst PWID through reducing the further transmission of HIV to their sexual partners. The impact could be even more than what we observed just for PWID, themselves. Internationally, what is reported as one measure of NSP coverage is the number of new syringes distributed per injector per annum, which is misleading. A more individual based measure, which can truly indicate the gap, is individual NSP converge. Such gap even exists amongst NSP service attendees. This has also been also reported in other studies, even in developing countries like Australia (16). The reasons for this gap are not clear and need to be further investigated. Providing more syringes per visit seems to work, but other reasons should not be neglected. In summary, our analysis highlights that NSP can be effective in a high prevalence setting. However, for harm reduction interventions to substantially improve the epidemic situation, they need to increase their coverage to higher levels than what was attained in Kermanshah, Iran. This could be done by encouraging more frequent visits, increasing hours and locations, and providing more syringes per visit (20, 21, 37). Although education and counseling is designed to reduce syringe sharing, increasing syringe coverage through NSPs is likely to be the most effective strategy to reduce incident infections. Increasing syringe coverage aims to decrease the number of times each syringe is shared and reduce the frequency of sharing (38, 39). Iran has been able to effectively introduce NSPs and increase the coverage of sterile injecting equipment.