1. Introduction
Chronic substance use produces significant deficits in executive control functions (ECF) and presents with several behavioral problems including apathy, lack of initiative (1), poor judgment, impulsivity (2), goal-neglect and disorganized behavior (3), and likewise leads to cognitive dysfunction by affecting attention, memory (4), intellectual functioning (5), learning, problem solving and perceptual motor speed (6, 7).
In particular, an inability to inhibit response has been identified as a core trait in addicted individuals (3, 4, 8-14) that similarly reflects in compulsive drug seeking and drug self-administration, even when the drug is no longer pleasurable (14-17). Repeated dopaminergic activation of the neural circuits by chronic drug use and subsequent neural dysfunction has been suggested to eventually produce a deficit in control over behavioral impulses (11, 14, 17). The prefrontal cortex (PFC) is the key component that is thought to underlie inhibitory control (18-23) and has recently been implicated in the reward circuit and addiction (3, 16, 24-26).
Methadone maintenance treatment (MMT) is one of the most frequently used opioid substitution treatments for drug addiction. MMT reduces harm associated with drug use and provides the stability necessary for the addict to change their lifestyle (27, 28). Despite the strong evidence on the effectiveness of opioid substitution therapy, there are reports that confirm the adverse effects of chronic methadone treatment on psychomotor and cognitive performance in these patients including processing speed, attention, working memory, short-term memory and decision making (29-34). However, these studies compared the cognitive performance between methadone maintenance patients (MMPs) and healthy controls. Comparing MMPs with drug abusers who are not yet enrolled in opioid substitution therapy may yield more accurate findings about the level and extent of methadone treatment’s influence on cognitive performance measurements.
2. Objectives
This study, therefore, included opiate users, along with MMPs and healthy controls, to detect the possible neuropsychological differences between MMPs and opiate users. We employed the Go/No-Go paradigm to evaluate and compare healthy participants, opiate abusers and MMPs in terms of inhibition response, which is proposed to be one of the core behavioral disruptions in chronic addiction.
3. Patients and Methods
3.1. Participants
Forty-five opiate-dependent individuals, 50 methadone maintenance patients (MMPs) and 50 healthy participants were recruited into this study through notices posted on an announcement board or by a verbal offer at the Iranian national center for addiction studies (INCAS) over the period of one year. Opiate-abusers were chosen based on the following criteria: 1) meeting the DSM-IV criteria for substance dependence; 2) having no history of any neurological or psychiatric disorders or any current medical illness; 3) self-reporting of opiate abuse (opium, heroin) purely, without abusing other substances such as stimulants or benzodiazepines, confirmed by a five-panel rapid urine test (COC/mAMP/OPI/THC/BZO) at admission. Among the opiate dependents, the number of years of opiate use was 12.2 (1 - 22 years), and the preferred method of abuse was reported as: intranasal (6%), smoking (42%), oral (44%) and intravenous injection (8%).
Fifty opioid-dependent MMPs were recruited from outpatient methadone maintenance programs in the addiction treatment clinic at the Iranian national center for addiction studies (INCAS) who fulfilled the following criteria: 1) being involved in a formal methadone maintenance treatment program; (b) being stabilized in their current methadone dose for at least one month; and (c) a minimum abstinence period of 48 hours from any drug except methadone, verified by urine screening. At the time of testing, none of the participants were experiencing withdrawal symptoms. The mean time of methadone maintenance therapy (MMT) was 12.2 ± 2.8 months and the mean dose was 77.6 ± 44.6 mg/day.
Fifty healthy participants were subsequently selected to be matched with the study groups in terms of age, gender and education level and who met the conditions that: 1) they had not taken any substances (excluding alcohol (at low level) or cigarette smoking) in the past; 2) they had no history of psychiatric or neurological disorders or were not on any medication for medical diseases.
The demographic data of the three groups are presented in Table 1. All the subjects provided written informed consent approved by the institutional review board (IRB) of Tehran University of Medical Sciences (TUMS), and thereafter, they were tested with six versions of Go/No-Go tasks.
| Controls, n = 57 | MMPs, n = 50 | Addicts, n = 45 | P Value | |
|---|---|---|---|---|
| Age, y | 33.7 ± 1.1 | 34.0 ± 1.1 | 36.3 ± 1.3 | 0.233 |
| Male gender (%) | 38 (66.7%) | 40 (80%) | 35 (77.8%) | 0.237 |
| Education, y | 9.6 ± 0.5 | 10.5 ± 0.5 | 9.0 ± 0.4 | 0.101 |
aData are presented as Mean ± SD or No. (%).
3.2. Procedure
This study was conducted in a condition-controlled room at the neurocognitive laboratory of the Iranian national center for addiction studies (INCAS). Each subject came separately to the room and was seated in a comfortable chair in front of the computer screen. After filling out the demographic information form, the subject received instructions to respond by pressing the space bar as quickly as possible to certain stimuli (Go stimuli) that were briefly displayed, one by one, in the center of the dark screen. In addition, they were instructed to withhold responses to other stimuli (No-Go stimuli). Each subject conducted the six variants of the Go/No-Go (GNG) tasks in a sequential order, and before each run, they were given a short break to receive instructions on the new target and distractor stimuli.
3.3. Measurements
In the neuro-cognitive laboratory of the Iranian national center for addiction studies (INCAS), six versions of Go/No-Go tasks (V1–V6) were provided based on a classic Go/No-Go paradigm in which subjects have to respond as quickly as possible to target stimuli and withhold responses to distractor stimuli. All seven versions of Go/No-Go tasks were developed using E-Prime V.2 software.
In V1 to V4, the Go stimuli were blue circles and the No-Go stimuli were yellow circles, while in V5 the target stimulus was the “O” sign and the No-Go stimulus was the ‘X’ sign. In V6, the target stimulus was the appearance of colored circle/s at the upper left and lower right, and the No-Go stimulus was the appearance of colored circle/s at the lower left and upper right. Each version consisted of an 80-stimulus presentation (trials) displayed one by one in a predefined fixed order. The ratio of Go to No-Go stimuli was 20% in all versions except V4, which had a target probability of 50%. Stimulus presentation time (SPT) is defined as the duration of stimuli appearance on the screen, which varied from 1,200 ms in V1 to 300 ms in the other five versions, while the inter-stimulus intervals (ISI) varied from 900 ms to 1,200 ms in different versions. Detailed characteristics of these six different versions are presented in Table 2.
| Variants | Go Stimulus | No-Go Stimulus | SPT, ms | ISI, ms | No-Go/Go Ratio | Number of Trials | Stimulus Position |
|---|---|---|---|---|---|---|---|
| V1 | • | • | 1200 | - | 1/4 | 80 | Center |
| V2 | • | • | 300 | 900 | 1/4 | 80 | Center |
| V3 | • | • | 300 | 1200 | 1/4 | 80 | Center |
| V4 | • | • | 300 | 900 | 1/1 | 80 | Center |
| V5 | O | X | 300 | 900 | 1/4 | 80 | Center |
| V6 | • | • | 300 | 900 | 1/4 | 80 | Center |
Abbreviations: ISI, inter-stimulus interval; SOI, stimulus onset interval.
The number of responses to targets (hits) and the number of “no” responses to non-targets (stops) are the two main measurements that the GNG task yields. “Hits” are the number of targets that are correctly detected through Go-trials, and “stops” are the number of non-targets that are accurately rejected. There are three other scores derived from these “hit” and “stop” scores: “omission errors (misses)” defined as the number of Go stimuli that were mistakenly missed; “commission errors” indicate the number of No-Go stimuli that are falsely responded to and could be regarded as the marker of disinhibition; “total true score” is the sum of “hits” and “stops” and shows the total number of true responses to both Go and No-Go stimuli. The number of “hits” can be regarded as a measure of behavioral initiation, whereas “commission errors” can be considered as a measure of inhibitory response.
“Reaction time of hits” and “reaction time of commission errors” show the time interval in milliseconds (ms) between the appearances of a Go or No-Go stimulus and pressing the space bar. The GNG task measurements and their range of variability are summarized in Table 3.
| Variable | Definition | Range |
|---|---|---|
| Overall Score (OS) | True hits on Go and No-Go trials | 0 - 80 |
| Hit | True hits on Go trials | 0 - 64 (All), 0 - 40 (V4) |
| Stop | True inhibitions on No-Go trials | 0 - 16 (All), 0 - 40 (V4) |
| Omission error (Miss) | Missed Go trials | Similar to TGS |
| Commission error (Error) | False hits on No-Go trials (Disinhibition) | Similar to TNGS |
| Reaction time of Hits | Reaction time of true Go trials (ms) | 100 - 1000 |
| Reaction time of omission error | Reaction time of false No-Go trials (ms) | 100 - 1000 |
3.4. Statistics
The results are presented as mean ± SD (standard deviation) for the quantitative variables and are summarized by frequency (percentage) for the categorical variables. One-way analysis of variance (ANOVA) was used to explore group differences in age and education level, while group differences regarding gender were examined using a χ2 analysis. Go/No-Go scores were not normally distributed (as assessed by Kolmogorov-Smirnoff tests) and were analyzed using the Kruskal-Wallis test. Pairwise comparisons were conducted via the Mann-Whitney nonparametric test. The differences with P < 0.05 were considered statistically significant between groups. For statistical analysis, the statistical software SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL) was used.
4. Results
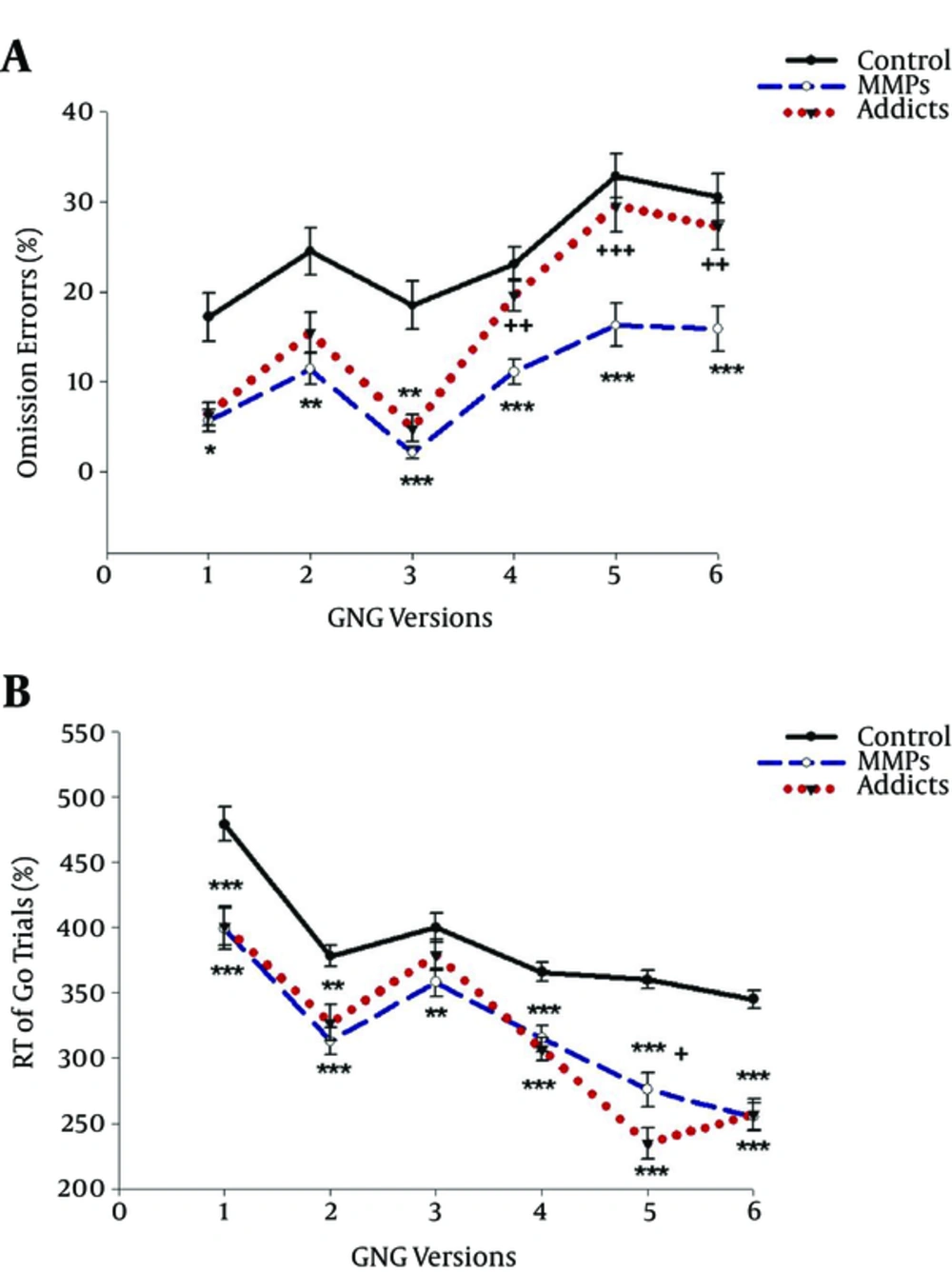
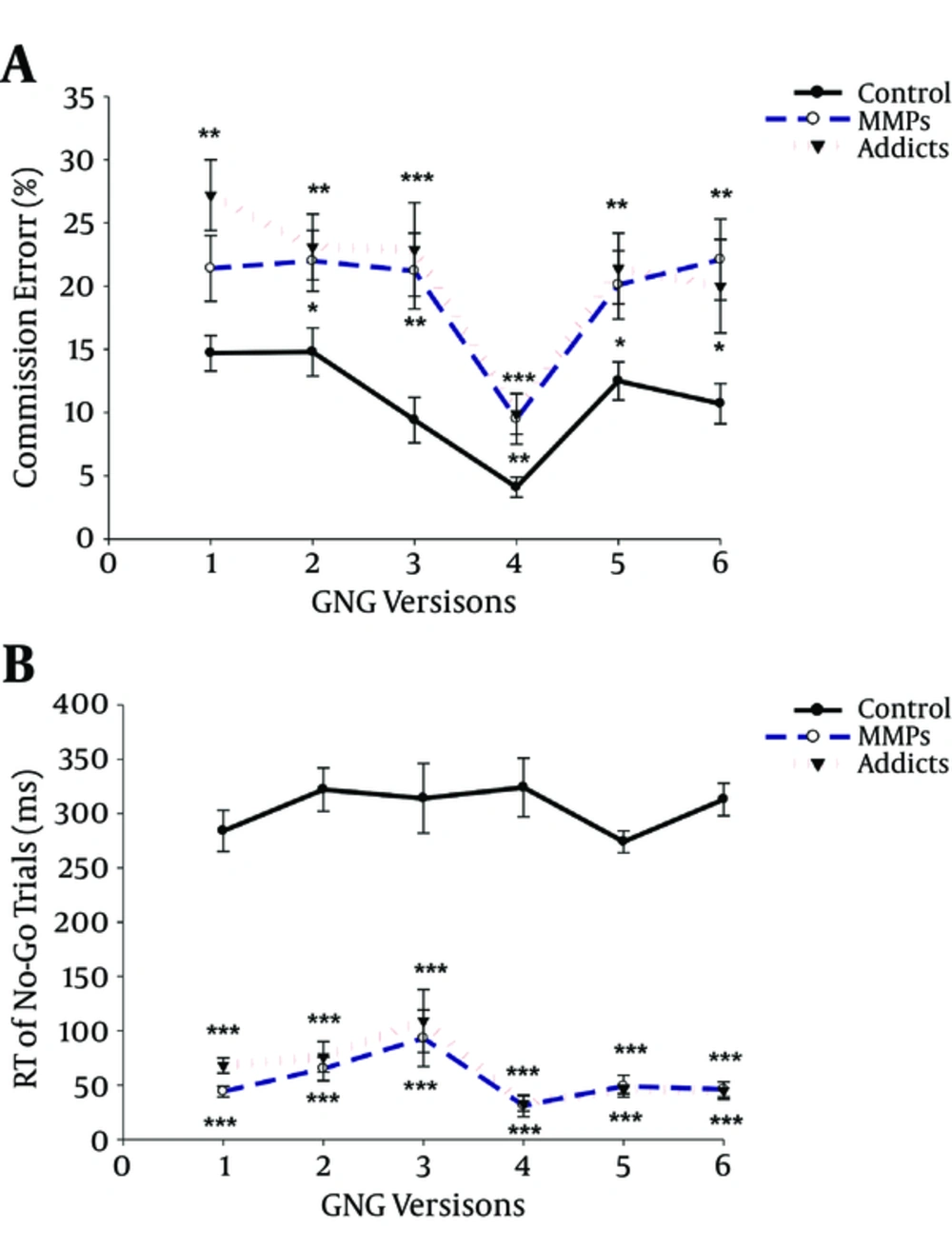
To simplify, all the Go/No-Go scores are presented as percentages in tables and figures. According to Table 1, all three groups, opiate dependents, MMPs and controls, were well matched on age, education and gender. As can be seen in Table 2, there is a significant difference between groups in terms of reaction time (RT) and errors in both Go and No-Go trials, with longer reaction time (RT) for both Go trials and No-Go trials in controls than opiate users and MMPs in all six versions.
Figures 1 and 2 reveal that controls omitted much more targets in Go trials than opiate abusers and MMPs, while they committed much fewer errors in No-Go trials in comparison to the other two groups in all six versions, indicating better response inhibition in normal controls.
Post-hoc analyses on No-Go trial scores as a measure of inhibition response did not show any significant difference on commission errors between opiate users and MMPs, suggesting that these two groups did not differ in terms of response inhibition. Regarding Go trials, there was no significant difference between opiate users and MMPs in terms of omission errors on V1 to V3, while opiate users performed significantly worse than MMPs on V4 to V6.
| Go Trial | P Value | No-Go Trial | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Controls | MMPs | Opiate Users | Controls | MMPs | Opiate Users | |||
| Version 1 | ||||||||
| Error | 17.7 ± 2.7 | 5.7 ± 1.2* | 6.4 ± 1.3 | 0.057 | 14.7 ± 1.4 | 21.4 ± 2.6 | 27.2 ± 2.8*** | 0.002 |
| Reaction time | 479 ± 13 | 399 ± 16*** | 401 ± 15*** | 0.000 | 284 ± 19 | 44 ± 5*** | 68 ± 7*** | 0.000 |
| Version 2 | ||||||||
| Error | 24.5 ± 2.6 | 11.4 ± 1.7** | 15.5 ± 2.2 | 0.003 | 14.8 ± 1.9 | 22 ± 2* | 23.1 ± 2.6** | 0.016 |
| Reaction time | 378 ± 8 | 313 ± 10*** | 327 ± 14** | 0.000 | 322 ± 20 | 65 ± 11*** | 76 ± 14*** | 0.000 |
| Version 3 | ||||||||
| Error | 18.5 ± 2.7 | 2.1 ± 0.7*** | 4.8 ± 1.5** | 0.000 | 9.4 ± 1.8 | 21.2 ± 3.0*** | 22.9 ± 3.7** | 0.000 |
| Reaction time | 400 ± 11 | 358 ± 11** | 379 ± 12 | 0.008 | 314 ± 32 | 93 ± 26*** | 109 ± 29*** | 0.000 |
| Version 4 | ||||||||
| Error | 23.1 ± 1.9 | 11.1 ± 1.4*** | 19.6 ± 1.8+++ | 0.000 | 4.1 ± 0.8 | 9.5 ± 2.0** | 9.9 ± 1.6*** | 0.000 |
| Reaction time | 366 ± 7 | 315 ± 10*** | 307 ± 9*** | 0.000 | 324 ± 27 | 31 ± 10*** | 33 ± 7*** | 0.000 |
| Version 5 | ||||||||
| Error | 32.9 ± 2.5 | 16.3 ± 2.4*** | 29.6 ± 2.9+++ | 0.000 | 12.5 ± 1.5 | 20.1 ± 2.7* | 21.4 ± 2.8** | 0.015 |
| Reaction time | 360 ± 7 | 276 ± 13*** | 235 ± 12***+ | 0.000 | 274 ± 10 | 49 ± 10*** | 46 ± 4*** | 0.000 |
| Version 6 | ||||||||
| Error | 30.5 ± 2.6 | 15.9 ± 2.5*** | 27.3 ± 2.6++ | 0.000 | 10.7 ± 1.5 | 22.1 ± 3.2** | 20.0 ± 3.7* | 0.012 |
| Reaction time | 345 ± 7 | 255 ± 11*** | 257 ± 12*** | 0000 | 313 ± 15 | 46 ± 7*** | 45 ± 8*** | 0.000 |
5. Discussion
Compared to controls, opiate users and MMPs showed poorer performance on the No-Go trials of a GNG task, attributable to the higher number of commission errors. No significant difference was found between opiate users and MMPs in terms of No-Go scores. The results revealed the subtle deficits in the ability of opiate users and MMPs to inhibit response against normal controls but did not show significant differences between opiate users and MMPs.
Consistent with current results, previous studies, also have demonstrated that substance dependent individuals, particularly cocaine users, present a higher number of commission errors in tests of response inhibition, such as a Go/No-Go task (9, 10, 35) or choice reaction time (RT) task (11) when compared against healthy controls. On the other hand, MMPs are shown to be significantly more impaired on measures of psychomotor performance, processing speed, working memory and short term memory (32, 34), and attention and visual orientation (33) than controls or abstinent heroin abusers (29). Inhibition behaviors have not been studied in MMPs as much as other aspects of executive function; however, Minitzer et al. suggested possible impairment in inhibitory mechanisms measured via the Stroop color-word paradigm compared to control participants (34).
Regarding Go-trials, our results surprisingly indicate that opiate users, and particularly MMPs, performed better and missed less target stimuli (less omission errors) than controls. This difference between drug users and controls might be attributable to differences in the reaction time (RT). As we see from the results, the RT of both Go and No-Go trials was significantly lower in opiate users and MMPs compared to controls, indicating that these individuals are faster to respond to a stimulus against healthy participants. Thus, it could be deduced that the probability of missing a target stimulus diminishes as RT decreases, because of the limited time available to respond to the stimulus. Consistently, Specka et al. reported that MMPs were faster but produced more errors compared to controls on a choice RT task (33). Here, it should be noted that the Go trials scores could not be interpreted by themselves as independent measures, since Go stimuli were intended to create a prepotent tendency to respond, which then necessitates inhibition with the appearance of a No-Go stimulus, and thus, the No-Go score is regarded as an exclusive score for the inhibitory process.
In conclusion, opiate users and MMPs seem to have a lower ability to control their responses than healthy controls; however, no significant difference was found between opiate users and MMPs on this measure of inhibition. The duration of methadone maintenance therapy (MMT) and the prescribed daily dose may affect the level of response inhibition behavior. Likewise, evaluation of individuals before and after starting opioid substitution therapy may aid in future investigations to better elucidate the possible and dose dependent effect of MMT on inhibition behavior.