1. Background
Methadone is long-acting synthetic opium used as an alternative to treat drug dependence and chronic pain (1). When administered orally, it is absorbed rapidly and completely and is detected in plasma about 30 - 60 minutes after oral administration (1). The half-life of the drug is 22 - 36 hours up to 59 - 90 hours, and therefore, in pain relief, it is superior to other opium, such as morphine and fentanyl, with half-life of 2 to 3 hours and 4 hours, respectively (1).
It is a fat-friendly drug widely spread across tissues, such as the brain, intestines, kidneys, liver, muscles, and lungs (2). Given that tissue distribution is superior to plasma protein binding capacity, the actual distribution of this substance during equilibrium is much higher than the plasma level, which varies depending on the patient's characteristics (3). On the other hand, methadone is highly bound to plasma proteins and mostly to alpha-glycoprotein, an essential part of it (up to about 86%) (4). Since this glycoprotein is a plasma protein that is increased in acute phase reactions, this could be the reason for the variety of plasma concentrations of the drug, particularly in cancer patients (4). Clinically, these pharmacokinetic properties increase the concentration of methadone in tissues after being administered several times, and therefore, the risk of overdose increases (5).
Despite the potential benefits of methadone, the consuming dose of this drug can be challenging for specialists. In particular, given the lack of a valid analgesic conversion ratio for methadone, the increased potential of this substance in patients who have previously used moderate to high doses of another opioid, high interpersonal diversity in pharmacokinetics, and numerous drug interactions, has made the standard use of methadone difficult (5).
Methadone pharmacology shows the lethal and hazardous effects of poisoning with this substance (6). The classic poisoning symptoms are central nervous system (CNS) depression, respiratory depression, and myositis, which could last for several days due to the long-term effects of methadone (7). Approximately 25% of deaths with methadone poisoning have been reported in the first 14 days of the methadone maintenance treatment with opioids (8). The plasma concentration between those who received the same dose of this substance can be very different (6). The pharmacokinetic diversity of this substance is most likely caused by the varied activity of cytochrome P450 enzymes responsible for metabolism. The diversity in CYP 3A4 activity is considerable (up to 400 times), which can be due to drug interactions or genetic differences in enzyme expression (9). The tolerance to sedative effects generally occurs after a few days; nevertheless, tolerance to analgesia occurs after days or months (10).
Vitamin C, also known as L-ascorbic acid, is a water-soluble vitamin found naturally in some foods (11). Recent studies are investigating the role of vitamin C in limiting the harmful effects of free radicals through antioxidant activity in preventing or delaying the progress of some cancers, cardiovascular diseases, and other diseases in which oxidative stress is involved (11). In the biosynthesis of neurotransmitters and neuropeptides, vitamin C has a significant impact on opioid-induced analgesia and dependence (12). Vitamin C is metabolized in the liver and small intestine and is excreted in urine and feces. Additionally, when the urine pH is above 6, renal excretion is responsible for only 4% of the total excreted drug; however, in pH lower than 6, 30% of the total dose is excreted by the kidneys (13). Studies have shown that vitamin C administration reduces the pH of urine (14, 15).
2. Objectives
Considering that acidic urine increases methadone excretion and whereas vitamin C is a low-risk with low interaction with other drugs (16), it is important to use other treatments in addition to naloxone. This study aimed to investigate the effect of vitamin C on the serum levels of methadone and urine methadone excretion.
3. Patients and Methods
3.1. Design and Setting
This was a single-blind randomized clinical trial carried out in Mostafa Khomeini Hospital in Ilam, southwest Iran, between July 2020 and June 2021. The study was approved by the Ethics Committee of Ilam University of Medical Sciences (IR.MEDILAM.REC.1399.106) and also was registered with the Iranian Registry of Clinical Trials (registration No.: IRCT20200621047863N1).
3.2. Sample Size
Taking into consideration the type of analysis and the possibility of interaction between intervention and time, α = 0.05, power = 0.80, number of measurements = 2, effect size = 0.22, and C = 7.9 and the fact that the study was conducted in three groups, the total required sample size was estimated to be 54 patients using G*Power software (17).
n = C × [(P1(1-P1) + P2(1-P2)/(P2–P1)2]
3.3. Inclusion and Exclusion Criteria
All patients who were referred to the hospital during this period were included in the study. Additionally, the patients with a history of kidney stones, hemochromatosis, glucose-6-phosphate dehydrogenase (G6PD), iron overload, and diabetes (given the impossibility of using high doses of vitamin C) were excluded. Moreover, the patients who used drugs or poisons other than methadone were excluded due to the possibility of a need for urinary alkalization. Moreover, one of the exclusion criteria in this study was death within 24 hours.
3.4. Sampling and Random Assignment
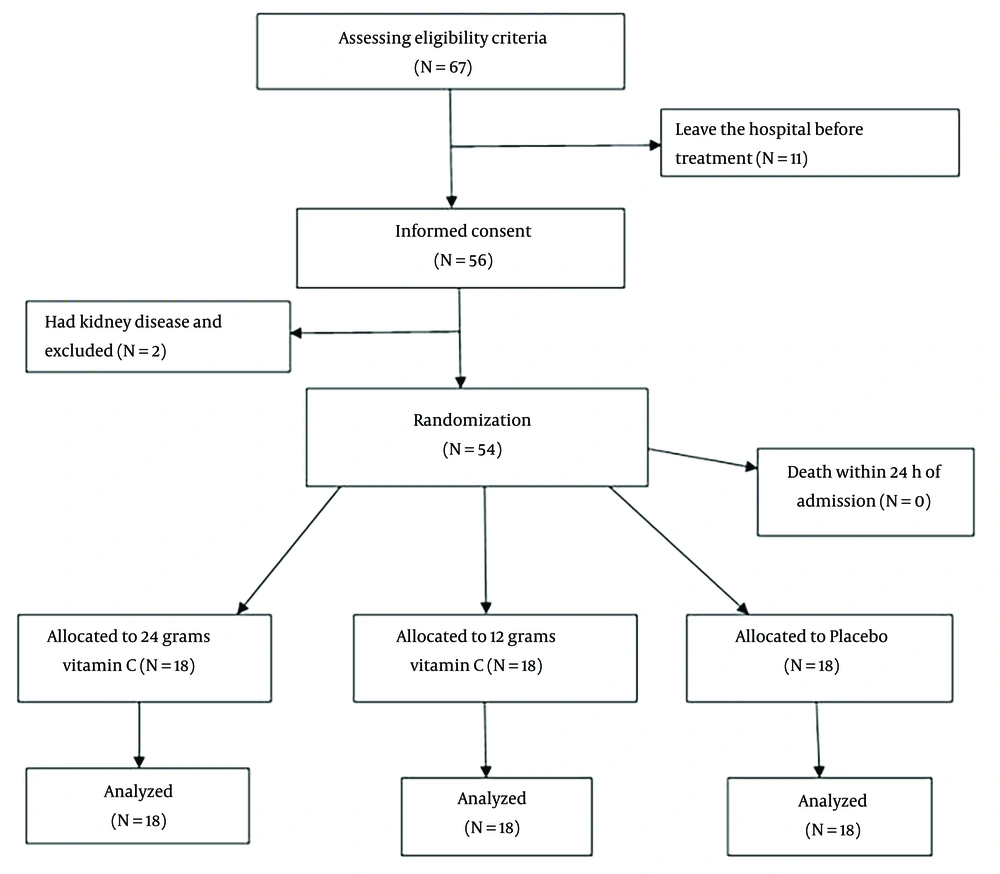
Sixty-seven patients diagnosed with methadone poisoning were admitted to the hospital during that period. Eleven patients chose to leave the hospital before treatment, and two patients with kidney disease were excluded from the study. Informed consent was obtained from the patients or their first-degree companions. Finally, 54 patients were randomly assigned to three groups (18 subjects per group), where two groups received two different doses of vitamin C (12 and 24 g), and the control group received usual care using simple randomization methods (Figure 1).
3.5. Intervention and Blinding
Up to 1.5 g of vitamin C was used per kilogram of body weight as a study for the anti-tumor effect, and no major complications were observed; therefore, these doses have no major side effects (13). In this study, 18 patients received 12 g of intravenous vitamin C (6 ampoules of 5 mL containing 500 mg of vitamin C mixed in 100 mL normal saline every 6 hours) for one day, 18 patients received 24 g of intravenous vitamin C (12 ampoules of 5 mL containing 500 mg of vitamin C mixed in 100 mL normal saline every 6 hours) for one day, and 18 patients only received 100 mL normal saline as the control group. All patients were restricted from eating and drinking and received the usual care and treatment according to their clinical needs. The attending physician, who was responsible for the patient's life, was aware of the treatment of the patients' groups. The patients, the laboratory staff, and the person who reviewed the results were blinded to the treatment, and the study was single-blinded.
3.6. Measurement
Baseline variables, such as age, gender, and methadone dosage, were measured in groups. Venous blood samples and urine specimens were collected from all patients on admission and 24 hours after admission. To measure the serum methadone level, the high-performance liquid chromatography (HPCL) method was used. The thymol blue and methyl red markers were also used in the urine strip to determine the urine pH.
3.7. Statistical Analysis
Statistical analysis was performed using STATA version 12. The Shapiro-Wilk test was used to examine the normality of data distribution. Differences in the baseline variables were compared between the three groups. There were no significant differences between the three groups for baseline variables. Differences in mean scores were investigated over two points of time. One-way repeated measure analysis of variance (ANOVA) was performed to compare the effect of the intervention, and the Bonferroni test was utilized to calculate pair-wise comparisons. The statistically significant level was considered 0.05.
4. Results
4.1. Baseline Data Analysis of Groups
There were no missing data for patients, and all quantitative variables had a normal distribution. The mean age of the total patients was 35.44 years (standard deviation [SD] = 14.72, range = 18 - 80). In the total sample, 37 (68.5%) and 17 (31.5%) subjects were male and female, respectively. The average methadone consumption in the patients was 17.78 cc (SD = 11.10, range = 5 - 40). The comparison between groups in terms of the baseline variables showed no significant difference between the three study groups regarding age variable (P = 0.09), gender variable (P = 0.66), and methadone dosage consumption variable (P = 0.46) (Table 1).
| Characteristics | Group | P-Value | ||
|---|---|---|---|---|
| Control | 12 g Vitamin C | 24 g Vitamin C | ||
| Total | 18 | 18 | 18 | - |
| Age,y | 31.22 ± 3.40 | 33.67 ± 2.56 | 41.44 ± 4.00 | 0.09 |
| Gender | 0.66 | |||
| Male | 11 (29.73) | 12 (32.43) | 14 (37.84) | |
| Female | 7 (41.18) | 6 (35.29) | 4 (23.53) | |
| Dose, cc | 16.11 ± 2.34 | 17.22 ± 3.08 | 20.00 ± 2.43 | 0.46 |
a Quantitative and qualitative variables were presented as mean ± SD or No. (%), respectively. Quantitative and qualitative variables were compared between three groups using one-way ANOVA and Fisher’s exact tests, respectively. The significance level was considered as 0.05.
4.2. Within-Subjects for Serum Methadone Level and Urine pH
The comparison of serum methadone level and urine pH in two-time measurements is shown in Tables 2 and 3 for the three study groups. The average serum methadone decreased over time; accordingly, it was 0.50 ± 0.19 on admission and 0.27 ± 0.17 the 24 hours after admission; the two values were significantly different (P = 0.002). Furthermore, the average urine pH in the two measurements showed no change over time; accordingly, it was 5.94 ± 0.74 and 5.89 ± 0.82 in the first and second times, respectively, with no difference between the two values (P = 0.25) (Table 3).
4.3. Intervention (Between-Subjects Variable)
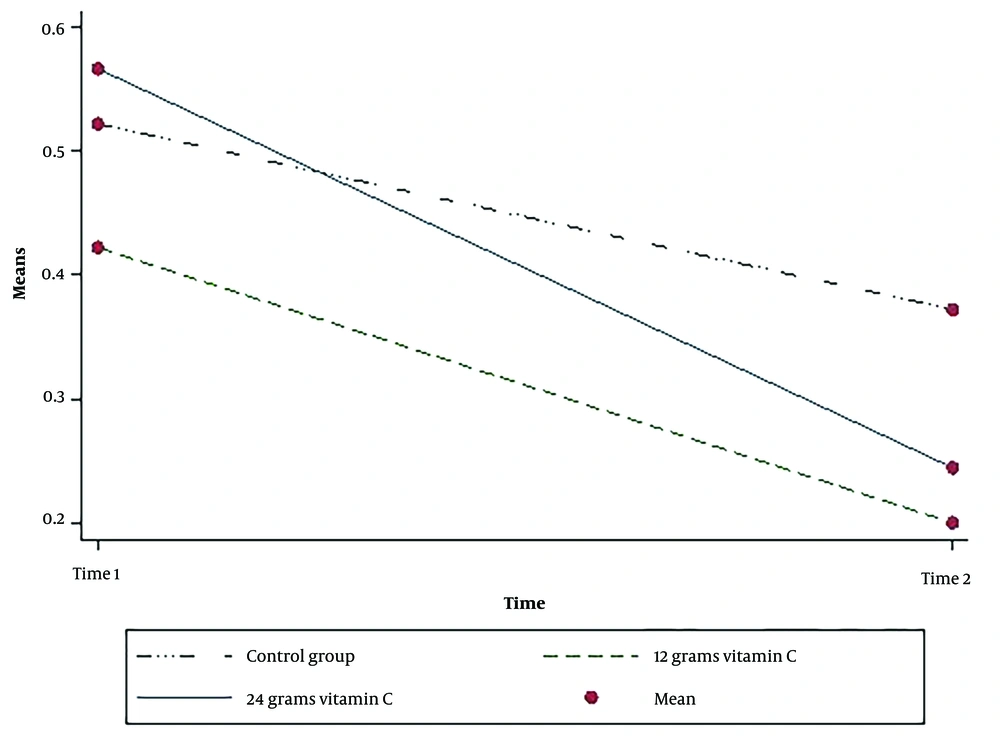
As observed in Table 3, the average serum methadone is reduced in the three groups; however, this decline in the two intervention groups was higher than in the placebo group, and these differences were statistically significant in the three groups after adjusting for age, gender, and methadone dosage consumed (P = 0.03). Bonferroni tests for pairwise comparisons showed that compared to the control, the serum methadone level was more significantly reduced after the consumption of 12 g of intravenous vitamin C (P = 0.04). Although the mean serum methadone decreased with the consumption of 24 g of vitamin C, compared to the control group; this difference was not statistically significant (P = 0.97).
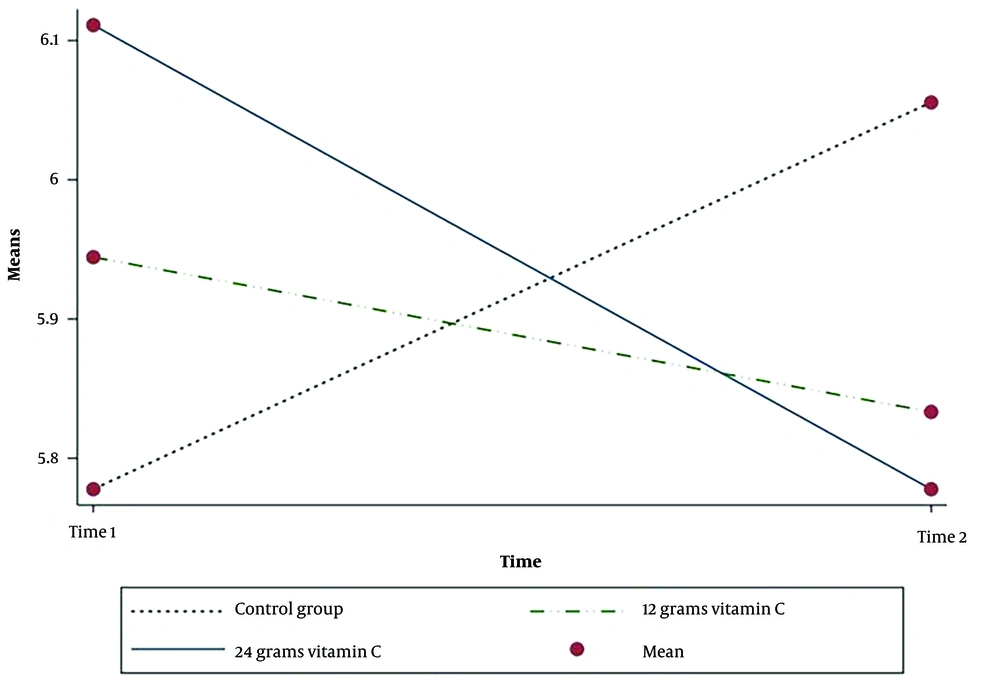
Therefore, according to the above-mentioned findings, 12 g of intravenous vitamin C considerably reduced the methadone level in the blood of patients poisoned with methadone. The post-intervention urinary pH was reduced in the two intervention groups; nevertheless, the urinary pH of the control group was increased (Table 2). However, these differences were not statistically significant in the three groups after adjusting for age, gender, and methadone dosage consumed (P = 0.98) (Table 3).
| Variables and Groups | On Admission, b | 24 h After Admission | Mean Change, c Time 2-Time 1 | P-Value |
|---|---|---|---|---|
| Serum methadone levels | ||||
| Control | 0.52 ± 0.19 | 0.37 ± 0.18 | 0.15 | 0.02 d |
| 12 g vitamin C | 0.42 ± 0.14 | 0.20 ± 0.11 | 0.22 | < 0.001 d |
| 24 g vitamin C | 0.57 ± 0.20 | 0.24 ± 0.18 | 0.32 | < 0.001 d |
| Urine pH | ||||
| Control | 5.78 ± 0.75 | 6.06 ± 0.66 | -0.28 | 0.25 |
| 12 g vitamin C | 5.94 ± 0.87 | 5.83 ± 0.98 | 0.11 | 0.72 |
| 24 g vitamin C | 6.11 ± 0.58 | 5.78 ± 0.81 | 0.33 | 0.16 |
a Values are expressed as mean ± SD.
b On admission (time 1); 24 h after admission (time 2).
c Variables were compared between three groups using a t-test.
d Significant
| Variables a | On Admission b | 24 h After Admission b | Total c | Between-Subjects d | Within-Subjects | Interaction Intervention × Time | ||
|---|---|---|---|---|---|---|---|---|
| Control (18) | 12 g Vit C (18) | 24 g Vit C (18) | ||||||
| Serum methadone level | 0.50 ± 0.19 | 0.27 ± 0.17 | 0.45 ± 0.19 | 0.31 ± 0.12 | 0.41 ± 0.18 | F = 3.73; ef = 2; eP = 0.03 f | gF = 10.97; ef = 1; P = 0.002 f | F = 8.66; ef = 2; P = 0.001 f |
| Urine pH | 5.94 ± 0.74 | 5.89 ± 0.82 | 5.92 ± 0.67 | 5.89 ± 0.88 | 5.94 ± 0.62 | F = 0.02; ef = 2; eP = 0.98 | F = 1.34; ef = 1; P = 0.25 | F = 3.39; ef = 2; P = 0.04 f |
a The mean ± SD studied variables were presented in two-time points.
b Average values of the dependent variables related to the between-subject values.
c Estimated marginal means related to the within-subject values.
d Repeated measure ANOVA model adjusted for age, gender, and methadone dose.
e Adjusted P-value
f Significant
g Between and within-subject analyses were performed using the Greenhouse-Geisser test.
4.4. Interaction Between the Time and Intervention in Serum Methadone and Urine pH
The results showed that there was an interaction effect between the group and time for serum methadone (P = 0.001) and urine pH (P = 0.04). There is strong evidence that the effect of intervention depends on time for two variables (Table 2) (Figures 2 -3).
5. Discussion
This study was performed to investigate the effect of intravenous vitamin C administration on the serum methadone level and urine methadone excretion in patients with methadone poisoning. The results showed that 12 g of vitamin C administration considerably reduces serum methadone levels. Although patients who received a total of 24 g of vitamin C had lower serum methadone levels, this difference was not significant. It seems that the higher average age and probably underreporting of the methadone dosage consumed by this group are the reasons for the non-significant results in this group. In the adjusted analysis, age did not affect the decrease of serum methadone; however, the amount of methadone dosage consumed showed a significant relationship with the serum methadone level (P < 0.05).
No study report was identified regarding the effect of vitamin C administration on serum methadone levels. However, some studies have shown that the intravenous administration of vitamin C decreases opioid withdrawal symptoms in humans (18, 19). In The current study, a statistically significant difference was not observed in the effect of vitamin C administration on urinary pH. In a study conducted by Noureldin et al., patients received an average of 1,000 mg of ascorbic acid per day for an average of 22.6 months, leading to a considerable reduction in the urine pH (14). Baxmann et al. showed that vitamin C supplementation caused urinary acidity (20).
In this study, different doses of vitamin C were given to the patients over a short period of time (24 hours). According to the above-mentioned studies, it seems that the long-term use of vitamin C is more associated with reducing urinary pH. Previous studies have shown that vitamin C has a low risk with low interaction with other drugs (21, 22). The results of a review study in 2003 cited rare complications caused by prescribing high intravenous doses of vitamin C, and only one case of tumor necrosis in a patient with cancer was found, followed by prescribing a high dose of vitamin C (21).
The results of a study by Murphy et al. showed that ascorbic acid is an effective urinary acid substance only in patients with non-contaminated urine (chronic urinary tract infections) (23). In patients with contaminated urine, ascorbic acid supplementation alone does not lead to a significant reduction in urinary pH (23). However, when ascorbic acid supplementation is combined with antibacterial treatment, such as nalidixic acid, melamine, or nitrofurantoin, it leads to a significant decrease in urine pH (23). In the study conducted by Nilsson et al., it was observed that with increasing urinary acidity, methadone excretion significantly increased. The results of the aforementioned study are consistent with the results of the present study regarding a decrease in methadone blood level and increased urinary acidity (24).
Bernard et al. showed that the methadone excretion in the urine depends on the pH of the urine, and the higher concentrations of methadone at lower pH levels are excreted in the urine (25). In a study by Ali Hassan et al., the long-term use of tramadol causes damage to the kidney’s glomeruli and long-term harmful consequences, such as proximal and distal convoluted tubule hypertrophy. The results of the aforementioned study showed that the intravenous administration of 100 mg/kg/b.wt of vitamin C to adult albino rats caused the improvement of distal and proximal convoluted tubules and the renal glomeruli (26).
Traxer et al. concluded that the intake of 2 g of ascorbic acid per day does not significantly change the urine pH in the patients. The results of the aforementioned study are in line with the results of the present study, which explained that vitamin C significantly does not reduce urinary pH (27). Vitamin C can be an effective and safe exogenous antioxidant that can reduce some of the side effects that come with acute and chronic opioid use. However, further investigation is necessary to comprehend the potential interactions with opioids, such as methadone toxicity.
5.1. Limitations
However, there were certain limitations in this study. Firstly, sometimes patients decide to leave the hospital before the end of the treatment protocol; to reduce this limitation, vitamin C should be prescribed immediately, and the patients should be informed of the dangers and unwanted consequences of early discharge from the hospital. The second limitation is the unknown type of poisoning in several patients with lower consciousness; to resolve this problem, faster toxicology screening should be considered. Thirdly, although positive results were observed in the group who received the 12 g of vitamin C in short-term follow-up, it seems the 24-hour follow-up duration was not sufficient to evaluate the effect of intervention groups. It is recommended that the results be validated by a long-term follow-up study.
5.2. Conclusions
This study indicates that the administration of 12 g of intravenous vitamin C reduces methadone blood levels in patients diagnosed with methadone poisoning. However, no significant effect of vitamin C on urinary pH was observed. Additionally, given the early discharge of patients from the hospital, the administration of a single dose of vitamin C in the patients could be investigated.