1. Context
Addiction is recognized as one of the major challenges, and according to the World Health Organization, 270 million people globally, including 2 million in Iran, are affected by addiction (1). About 0.9% of the global population and 3.5% of American adults suffer from non-alcoholic substance abuse. Stimulants, such as methamphetamines, are the second most commonly abused illegal drugs worldwide. According to the United Nations Office on Drugs and Crime, Iran ranks fifth in methamphetamine addiction (2, 3).
Studies have indicated that individuals with methamphetamine use disorder (MUD) exhibit deficits in motor function, social cognition, as well as executive functions, verbal learning, and memory (4). Research has addressed the cognitive deficit by linking it to a reduction in the activity of the dorsolateral prefrontal cortex (DLPFC), a region in the frontal cortex (5). Alizadehgoradel et al. (2) have shown that methamphetamine users exhibit significant deficits in working memory, cognitive flexibility, decision-making, and inhibitory response. Another study (6) also indicated that attentional control and verbal memory are weaker in methamphetamine users. Rezaee et al. (3) investigated the cognitive functions in methamphetamine users using the Wechsler Memory Scale (WMS-IV) and found significant differences in all subscales (e.g., logical memory, associative learning, visual memory). Jiang et al. (7) showed that individuals with MUD demonstrate higher impulsivity compared to healthy individuals.
Over the past few decades, various drug interventions have been attempted to reduce the effects of methamphetamine abuse and promote harm reduction. Despite some studies showing promising results, a recent systematic review indicated that psychostimulants do not have a significant effect on sustained abstinence or treatment retention (8). Other types of interventions, including motivational interviewing, cognitive behavioral therapy (CBT), behavioral activation, and exercise, can help maintain abstinence, but these interventions often have a high discontinuation rate (9).
Given the importance of the frontal cortex in cognitive impairments and the lack of sustained abstinence or treatment retention with current drug interventions, transcranial direct current stimulation can be used as a new approach in the treatment of MUD. Transcranial direct current stimulation delivers a weak direct electrical current to the brain cortex through the skull, contributing to the depolarization and hyperpolarization of the neural membrane. This method can modulate neural excitability and enhance brain flexibility. Today, tDCS is used in multiple psychiatric disorders, including mood disorders, obsessive-compulsive disorder, mild cognitive impairment, and dementia (10). It has been shown that by modulating brain activity, this approach may contribute to improving cognitive functions and reducing substance craving.
Jitendriya et al. (11) have demonstrated that bilateral tDCS in alcohol-dependent subjects activates the DLPFC and anterior cingulate cortex (ACC), facilitating abstinence from alcohol. In a clinical trial, Patel et al. (12) have shown that tDCS use in cannabis users is ineffective in improving cannabis craving and risk-taking behavior. A recent meta-analysis by Mehta et al. (13) identified ninety-four studies examining the effects of brain stimulation, including tDCS, on substance use outcomes (e.g., craving, consumption, and relapse) among individuals with substance use disorders, including alcohol, tobacco, cannabis, stimulants, and opioids. The analysis showed that right anodal DLPFC stimulation via tDCS produced a medium effect size for drug use and craving.
There have been some preliminary trials demonstrating the potential of tDCS in individuals with stimulant use disorder. A study investigated the effect of tDCS on the DLPFC for the impulsivity of individuals with MUD. Patients were divided into three groups: (1) an anodal tDCS group, (2) a cathodal tDCS group, and (3) a sham tDCS group, with a current intensity of 2 mA. The tDCS intervention was conducted twice a day for five consecutive days. The results showed that impulsivity was effectively reduced by the anodal tDCS intervention on the left DLPFC (14). Fayaz Feyzi et al. (15) examined the effectiveness of tDCS over the DLPFC in combination with Matrix Model psychotherapy in improving cognitive deficits and alleviating cravings in methamphetamine users. In this randomized, sham-controlled trial, participants were assigned to one of three groups: Matrix psychotherapy only, sham tDCS plus Matrix, or active tDCS plus Matrix. Sixteen sessions of 20-minute anodal tDCS at 2 mA over the left DLPFC were administered. The results showed that the active tDCS group experienced a greater reduction in cravings, and auditory and visual memory significantly improved in this group but not in the other groups. They also found a significant correlation between craving reduction and cognitive functioning in the active tDCS group.
Alongside these clinical trials, a 2023 systematic review and meta-analysis specifically focused on tDCS in MUD (9). To our knowledge, this is the first and only systematic review that identifies MUD research and focuses on the cravings and adverse effects of tDCS. As noted in the review, all included studies were conducted in Iran or China, indicating that studies published in non-English languages may have been missed. The review only focused on craving symptoms and did not consider cognitive functions.
2. Objectives
We conducted a bilingual search (English and Persian) and included any reported cognitive function outcomes. Given the importance of MUD and the low efficacy of biological interventions, it is useful to systematically explore the effect of tDCS in MUD by considering bilingual searches and cognitive deficits.
3. Materials and Methods
3.1. Search Strategy
A comprehensive literature search by two authors (S.M.S. S. and F.R.) was conducted using PubMed, Scopus, Web of Science, Google Scholar, and Science Direct databases through October 2023. Persian articles were searched on databases including Google Scholar, Scientific Information Database, and Noormags. A list of keywords and search terms was included: Methamphetamine, tDCS, addiction, craving, and cognitive function. These keywords were used in titles and abstracts (Table 1).
| Databases | Initial Search | Selection (Based on Title) | Selection (Based on Abstract) |
|---|---|---|---|
| Pubmed (English) | 137 | 18 | 10 |
| Scopus (English) | 272 | 55 | 2 |
| Web of science (English) | 25 | 3 | 0 |
| Google Scholar (English) | 50 | 0 | 0 |
| Science Direct (English) | 126 | 13 | 1 |
| Google Scholar (Persian) | 138 | 21 | 7 |
| SID (Persian) | 92 | 11 | 3 |
| Noormags (Persian) | 30 | 4 | 0 |
| Total | 870 | 125 | 23 |
3.2. Eligibility Criteria
Using PICOS (16), studies were included if they satisfied the following criteria:
Population (P): Studies recruiting participants (18+ years of age) diagnosed with MUD according to standardized criteria (e.g., DSM-IV or DSM-5); Intervention (I): Studies employing tDCS.
Comparison (C): Studies including either sham stimulation, a control group receiving no intervention, or an active control arm were included. Outcomes (O): Studies investigating substance-related outcomes (e.g., consumption, craving, cue-induced craving, abstinence, relapse) as primary outcomes, or cognitive measures (e.g., executive function, cognitive flexibility, attentional bias) as secondary outcomes. Study design (S): Studies employing either a parallel (between-subject) or cross-over (within-subject) randomized controlled trial (RCT).
Studies were excluded if they met the following criteria:
(1) Use of brain mapping or neuroimaging without presenting results of neurocognitive assessments.
(2) Non-reporting of substance use or the presence of individuals with substance use other than methamphetamine in the experimental group.
(3) Case studies and cross-sectional studies.
(4) Literature reviews, meta-analyses, dissertations, abstracts, conference presentations, or case reports.
3.3. Study Selection
Two authors (S. M. S. S. and F. R.) screened titles and abstracts to determine eligibility for full-text review and subsequently reviewed the full text of the selected studies. Disagreements were resolved by consensus and review with the senior authors (M. K. and A. S. H.).
3.4. Data Extraction
For included studies, the following data were extracted from the full texts: Author information, group characteristics (intervention and control, sample size, mean age), stimulation parameters (anode/cathode protocol, sessions, duration, and intensity), consumption characteristics (duration of use, weekly use frequency, daily consumption), primary substance use outcomes (e.g., craving and consumption), secondary substance use outcomes (e.g., executive function), the assessment instrument used, and assessment duration.
3.5. Risk of Bias
The quality of the included studies in the meta-analysis was assessed using the Cochrane Risk-of-Bias Tool (RoB-2) (17). Studies with a high risk of bias were excluded if two or more domains were flagged as high risk or if the overall risk of bias (ROB) was high.
3.6. Data Analysis
Since the studies used different scales, we calculated the effect size of changes in methamphetamine craving using the Standardized Mean Difference (SMD; Hedge’s g) with 95% confidence intervals (CIs) for each selected study that compared tDCS to sham intervention (P ≤ 0.05, two-tailed). Random effects models were used for individual MUD data from studies that reported end-of-treatment craving data from both active and sham tDCS arms. Negative values indicated that active stimulation produced greater reductions in craving, cue-induced craving, and/or consumption compared to sham treatment. The I² statistic was used to estimate between-trial heterogeneity, with I² values of ≤ 40% considered low heterogeneity, 40 - 60% moderate heterogeneity, and > 60% high heterogeneity (18). Meta-analyses were performed using Comprehensive Meta-Analysis Software (CMA) version 2.
4. Results
4.1. Search Results
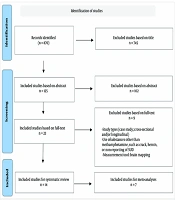
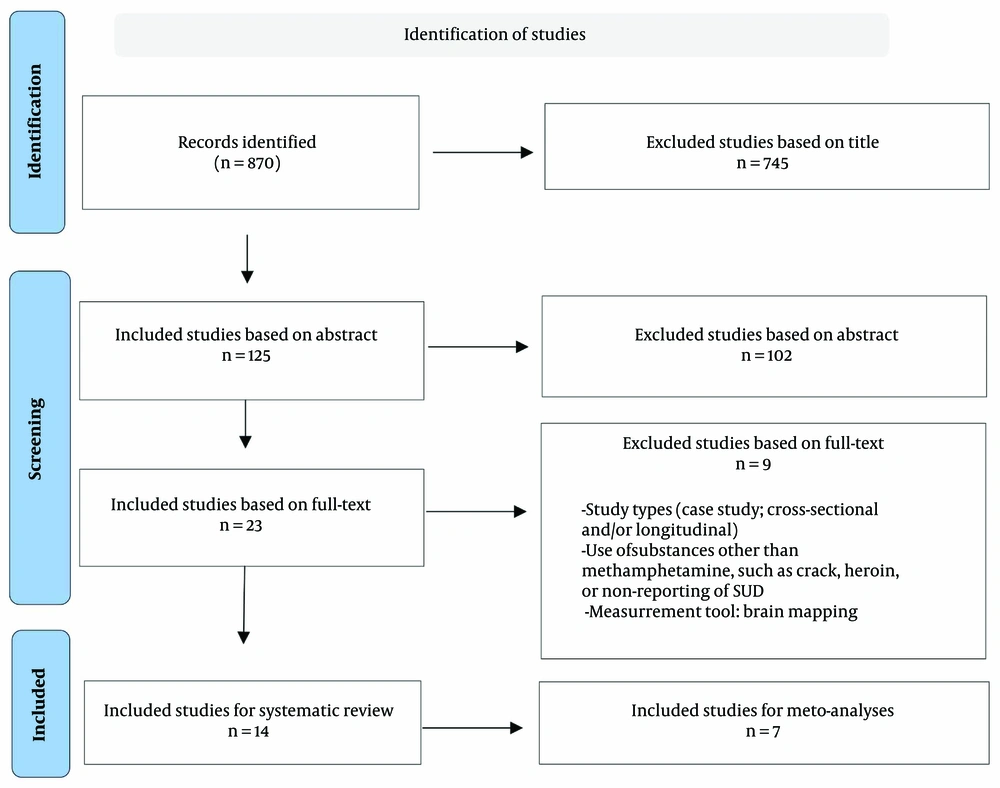
Following the initial search in the databases, a total of 260 articles were found in the Persian search and 610 articles in the English search. Based on the titles, 224 articles from the Persian search and 521 articles from the English search were excluded. In the Persian articles, 36 studies were reviewed based on their abstracts, and in the English articles, 89 studies were reviewed in the same manner. At this stage, 26 Persian articles and 76 English articles were excluded. Ultimately, a total of 23 full-text articles were examined. After a thorough review of the full texts, 9 articles were excluded, resulting in a final checklist review of 14 articles (Figure 1). The results of the search process and the number of articles identified at each stage, categorized by information databases, are presented in Table 1.
4.2. Study Characteristics
4.2.1. Demographic Data
The descriptive distribution of studies regarding age, the number of participants, and a history of methamphetamine use (if reported in the study) is presented for the two groups: Real tDCS and sham tDCS in Table 2.
| Variables | tDCS Group (n = 284) | Sham Group (n = 211) | Total (N = 495) |
|---|---|---|---|
| Age | 29.7 ± 6.64 | 30.49 ± 6.71 | 30.13 ± 6.67 |
| Participant | 18.46 ± 6.31 | 17.34 ± 6.42 | 17.92 ± 6.36 |
| Duration of meth use (y) | 4.8 ± 1.77 | 6.06 ± 4.3 | 5.42 ± 3.04 |
4.2.2. Domain and Scales of Measurements
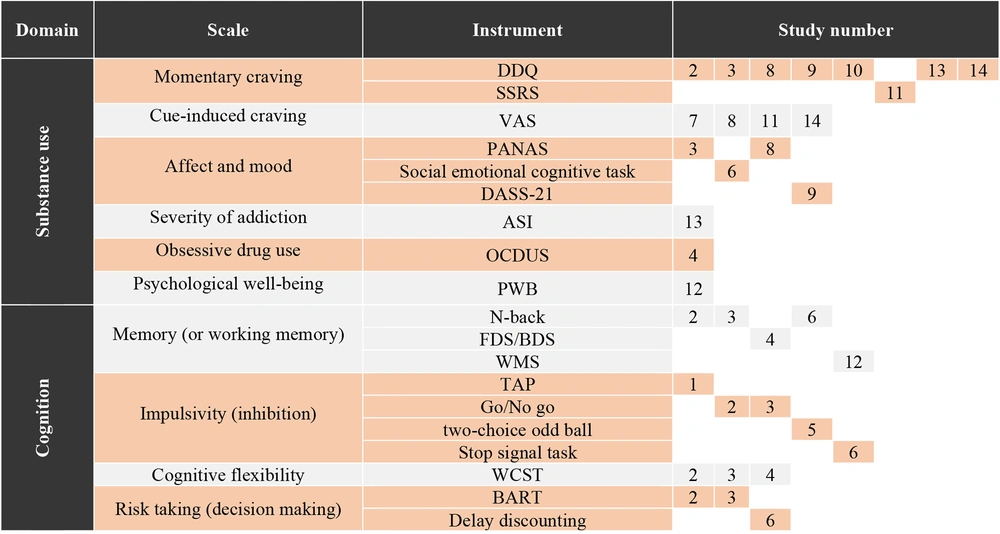
In reviewing the full texts of the articles, it was determined that evaluations related to the affected individuals can be summarized into two general domains: Substance use and cognitive factors. Upon examining the tools and measurement domains, it is evident that studies do not agree on the use of a uniform tool, resulting in heterogeneous assessments in both cognitive and substance use domains. Given this variation, descriptive information on the measurement domains is presented in Figure 2, categorized by the study number.
4.2.3. Treatments, Complementary Treatment, and Control Groups
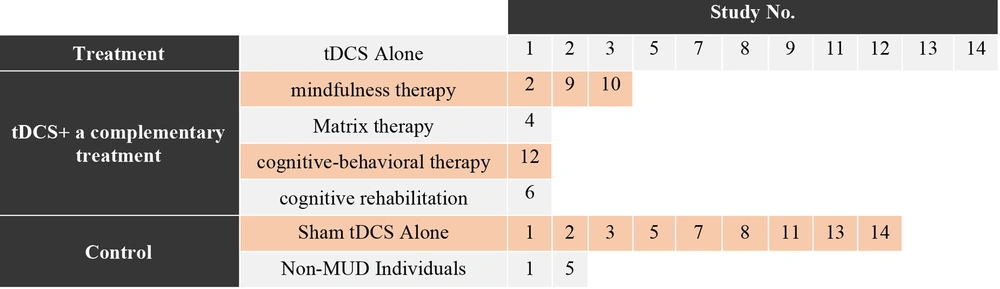
In all studies, at least one group involved tDCS. Out of the 14 reviewed studies, 11 studies had a real tDCS group without a combination with another simultaneous intervention (studies: 1, 2, 3, 5, 7, 8, 9, 11, 12, 13, and 14), and 9 studies included a sham tDCS group without a combination with another intervention (studies: 1, 2, 3, 5, 7, 8, 11, 13, and 14). Only two studies used a group of non-substance use disorder individuals as controls (studies: 1 and 5, Table 3). Several studies utilized complementary treatment methods simultaneously with electrical stimulation or as an independent intervention. Studies 2, 9, and 10 involved mindfulness therapy, while studies 4, 12, and 6 respectively incorporated Matrix therapy, cognitive-behavioral therapy, and cognitive rehabilitation (Figure 3).
| Author and Ref. | Group (Sample Size) | Mean Age (SD) | Consumption | Meta-analysis Inclusion |
|---|---|---|---|---|
| He et al. (14) | (1) Real tDCS group1 (n = 30); (2) real tDCS group 2 (n = 28); (3) Sham tDCS (n = 30); (4) healthy control (n = 30) | 29.89 (5.82) | NA | No |
| Alizadehgoradel et al. (2) | (1) Real tDCS (n = 16); (2) mindfulness (n = 15); (3) tDCS + mindfulness (n = 17); (4) Sham tDCS (n = 16) | 19.46 (1.18) | NA | Yes |
| Alizadehgoradel et al. (19) | (1) Real tDCS (n = 19); (2) sham tDCS (n = 20) | 34.83 (9.16) | DOU: 4.37, WUF: 6.21 (2.34); DOU: 4.35, WUF: 5.45(2.30) | Yes |
| Fayaz Feyzi, et al. (15) | (1) Real tDCS + psychotherapy (n = 15); (2) sham tDCS + psychotherapy (n = 12); (3) psychotherapy (n = 13) | 35 (7.23) | DOU: 5.4(2), DCA: 2.5(1.5); DOU: 4.5(1.3), DCA: 1.7(0.9); DOU: 5.9(2.4), DCA: 3(1.8) | Yes |
| Jiang et al. (7) | (1) Real tDCS (n = 23); (2) sham tDCS (n = 22); (3) Healthy control (n = 24) | 24.43 (2.97) | NA | No |
| Xu et al. (6) | (1) Real tDCS + CCR (n = 24); (2) sham tDCS + CCR (n = 26); (3) control (n = 23) | 33.57 (6.31) | DOU: 7.61 (4.57); DOU: 8.21 (5.54); DOU: 8.95 (5.24); | No |
| Ekhtiari et al. (20) | (1) Real tDCS (n = 23); (2) sham tDCS (n = 22); | 36.355 (8.35) | DOU: 9.67(8.92); DC: 1.74 (1/84); DOU: 16.44 (27.37); DC: 1.47 (1.3) | Yes |
| Rohani Anaraki et al. (21) | (1) Real tDCS (n = 15); (2) sham tDCS (n = 15) | 33.40 (11.87) | DOU: 4.7 (2.58); DOU: 5.06 (2.65) | Yes |
| Alizadehgoradel (22) | (1) Real tDCS (n = 20); (2) mindfulness (n = 15); (3) Real tDCS + mindfulness (n = 17); (4) Sham tDCS + mindfulness (n = 16) | 19.46 (1.18) | DOU: 3.6 (0.77); DOU: 3.13 (1.06); DOU: 2.88 (0/99); DOU: 2.87 (0.89) | No |
| Rahmani et al. (23) | (1) tDCS + Mindfulness (n = 15); (2) Usual addiction treatment (n = 15) | 39.11 (8.29) | NA | No |
| Sharifat et al. (24) | (1) Real tDCS (n = 15); (2) sham tDCS (n = 15) | 29.00 (2.97) | DOU: 2.3 (0.9); DOU: 3.8 (0.8) | Yes |
| Rezaee et al. (3) | (1) Real tDCS; (2) CBT; (3) sham tDCS; | NA | NA | No |
| Araghi and Ranjbar (25) | (1) Real tDCS (n = 10); (2) sham tDCS (n = 10); | 28.25 (5.81) | DOU: 5.2; DOU: 5.9 | No |
| Helmzadeh (26) | (1) Real tDCS 1 (n = 7); (2) real tDCS 2 (n = 7); (3) sham tDCS (n = 7) | 7 (NA) | NA | Yes |
Abbreviations, DOU, duration of use (years); WUF, weekly use frequency; DC, daily consumption (grams); CCR, computerized cognitive rehabilitation; CBT, cognitive-behavioral therapy; A, anode; C, Cathode; lDLPFC, left dorsolateral prefrontal cortex (F3); rDLPFC, right dorsolateral prefrontal cortex (F4); FC, frontal cortex.
4.2.4. tDCS Protocol Design
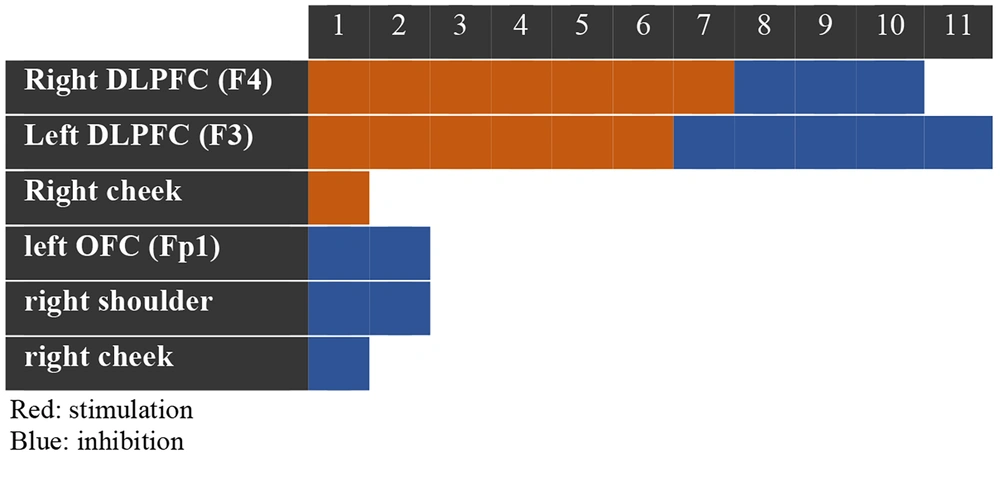
Regarding the protocols used in tDCS interventions, the placement of the stimulating electrode (anode) varied. Seven protocols focused on stimulating the right DLPFC, six on stimulating the left DLPFC, and one on stimulating the right cheek. The placement of the inhibitory electrode (cathode) showed more diversity: Five protocols inhibited the left DLPFC, three inhibited the right DLPFC, two inhibited the left Superorbitofrontal Cortex (Fp1), one inhibited the right shoulder, and one inhibited the right cheek (Figure 4). The study with the most sessions of brain stimulation was Study 6, consisting of 20 therapeutic sessions lasting 20 minutes each, conducted over 5 weeks with a current intensity of 2 mA. The fewest sessions were associated with Studies 7 and 11, which involved only one session lasting 20 minutes with a current intensity of 2 mA.
A summary of the reviewed studies, including group characteristics, assessment domains, duration, instruments, and intervention characteristics, is presented in Tables 3 and 4.
| Study (Ref.) | Cognitive Domain | Non-cognitive Domain | Assessment Duration | Instrument | tDCS Protocol | Sessions, Duration, Intensity |
|---|---|---|---|---|---|---|
| 1 (14) | NA | Aggressive behavior | Pre/Post-test | TAP (CRTT) | (1) A: F3, C: Right cheek; (2) A: Right cheek, C: F3; (3) A: F3, C: Right cheek | 5 consecutive days, twice a day; 20min, 2 mA |
| 2 (2) | Working memory; cognitive flexibility; decision making; inhibition response | Craving | Pre/post-test; 1-month follow-up | N-back*; WCST*; BART*; Go/No go*; DDQ* | A: F3, C: F4 | 12 sessions tDCS (2 sessions every week); 20 min, 2 mA; 12 sessions mindfulness (2 sessions every week); 45 to 50 min |
| 3 (19) | Working memory; cognitive flexibility; decision making; inhibition response | Affect; craving | Pre/post-test; 1-month follow-up | N-back*; WCST*; BART*; Go/No go*; DDQ*; PANAS* | A: F3, C: F4 | 10 sessions (2 sessions every week); 20 min, 2 mA |
| 4 (15) | Working memory; cognitive flexibility | Obsessive-compulsive drug using | Pre/post-test | FDS/BDS; WCST; OCDUS* | A: F3, C: left shoulder | 16 sessions (2 sessions every week); 20 min, 2 mA; 24 sessions of psychotherapy based on the matrix protocol. |
| 5 (7) | NA | Behavioral impulsivity | Pretest; after day 1; after day 5 | Two-choice odd ball | A: F4, C: F3 | 5 consecutive days; 20 min, 2 mA |
| 6 (6) | Attentional bias; inhibition response; decision making; verbal memory; working memory | Social emotions | Pretest; after 2 weeks; after 4 weeks | Dot probe task; stop signal task*; delay discounting*; ISLT; N-back; social emotional cognitive task | A: F4, C: F3 | 20 sessions (4 sessions every week); 20 min, 2 mA |
| 7 (20) | NA | Cue-induced craving | Pre/Post-test | VAS* | A: F4, C: Fp1 (left supraorbital) | Single session; 20 min, 2 mA |
| 8 (21) | NA | Affect; drug craving; cue-induced craving | Pre/post-test | PANAS; DDQ; VAS * | A: F4, C: F3 | 5 sessions; 20 min, 2 mA |
| 9 (22) | NA | Anxiety; stress; depression; drug craving | Pre/post-test; follow up | DASS-21*; DDQ* | A: F3, C: F4 | 12 sessions tDCS (72 hours intervals); 20 min, 1.5 mA; 12 sessions mindfulness (2 sessions every week); 45 to 50 min; mindfulness sessions were held immediately after tDCS. |
| 10 (23) | NA | Craving | Pre/post-test; follow up | DDQ*; RSP | NA | 10 sessions (twice a week); 20 min, 2 mA |
| 11 (24) | NA | Craving | before starting; 10 minutes after; End of session | SRRS; VAS* | A: Right FC, C: Left FC | Single session; 20 min, 2 mA |
| 12 (3) | Logical memory; mental control; orientation; personal and public informat ion; associative learning; visual memory; cognitive functions | Psychological well-being | Pre/post-test; follow up | WMS*; PWB* | NA | 12 sessions tDCS; 20min, 2 mA; 12 sessions CBT; 90 min |
| 13 (25) | NA | Craving | Pre/post-test | DDQ*; ASI | A: F4, C: Fp1 (left supraorbital) | 10 consecutive days; 20 min, 2 mA |
| 14 (26) | NA | Drug craving; cue-induced craving | Pre/post-test | DDQ*; VAS* | (1) A: F3; (2) A: F4 | 10 tDCS sessions; 20 min, 2 mA |
Abbreviations: TAP, Taylor aggression paradigm; CRTT, competitive reaction time task; WCST, Wisconsin card sort task; BART, balloon analoge risk task; DDQ, desire for drug questionnaire; PANAS, positive and negative affect Schedule; FDS/BDS, forward digit span. backward digit span; OCDUS, Obsessive-Compulsive Drug Use Scale; ISLT, International shopping list task; VAS, Visual Analog Scale; DASS, Depression; Anxiety and Stress Scale; RSP, Relapse scale prevention; SRRS, Stimulant Relapse Risk Scale; WMS, Wechsler Memory Scale; PWB, Psychological Well Being scale; ASI, Addiction Severity Index.
a * P < 0.05.
4.3. Meta-analysis
4.3.1. Quality Analysis
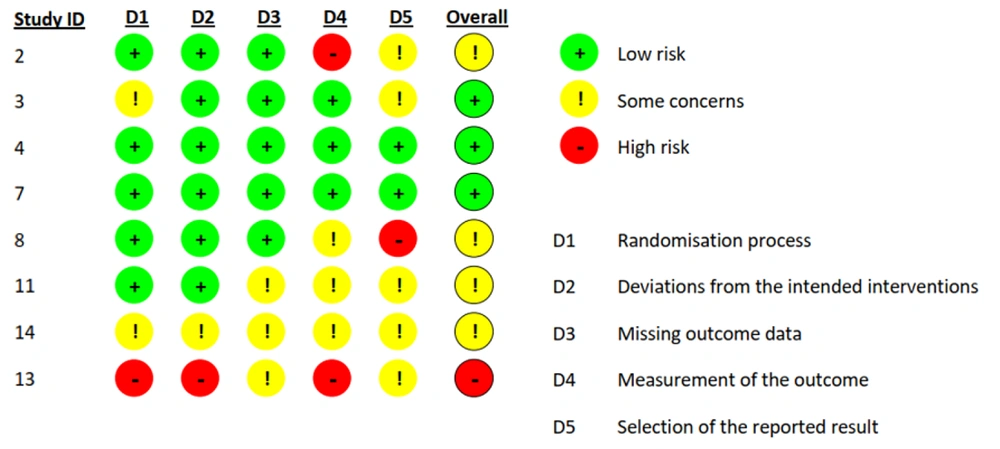
Overall, the quality of the studies was low, with the highest risk of bias found in the measurement of outcomes. See Figure 5 for the risk of bias assessment graph. Based on this assessment, we found that one study (Study 13) had a high overall risk of bias, so it was removed from the meta-analysis. We then considered the remaining seven studies based on their craving scores and calculated any scores reported on craving scales (e.g., desire and intention, negative reinforcement, and total).
4.3.2. Heterogeneity Analysis
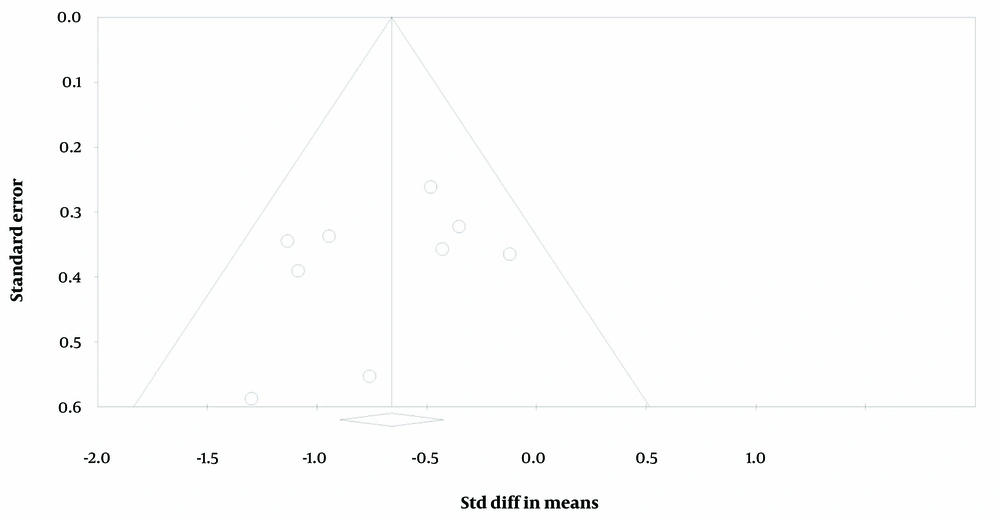
Initially, the meta-analysis revealed substantial heterogeneity among the included studies, with an I² value of 73.05%, indicating a high level of variability beyond what would be expected by chance. Cochran’s Q test supported this observation, showing a Q value of 4.53 with a significant P-value (P = 0.00), suggesting significant heterogeneity. The Tau² estimate was 0.416, reflecting considerable between-study variance. To address this high level of heterogeneity, we employed a funnel plot analysis to identify and exclude outlier studies (see Figure 6). After removing four items that contributed to the observed heterogeneity, the updated analysis showed a marked reduction in heterogeneity. The revised I² value was 10.71%, indicating low heterogeneity. Cochran’s Q value increased to 8.96, with a P-value of 3.46, suggesting that the remaining variability was no longer statistically significant. The Tau² was reduced to 0.126, indicating a decrease in between-study variance. These adjustments improved the consistency of the meta-analysis results and provided a clearer understanding of the effect sizes.
4.3.3. Meta-analysis Results
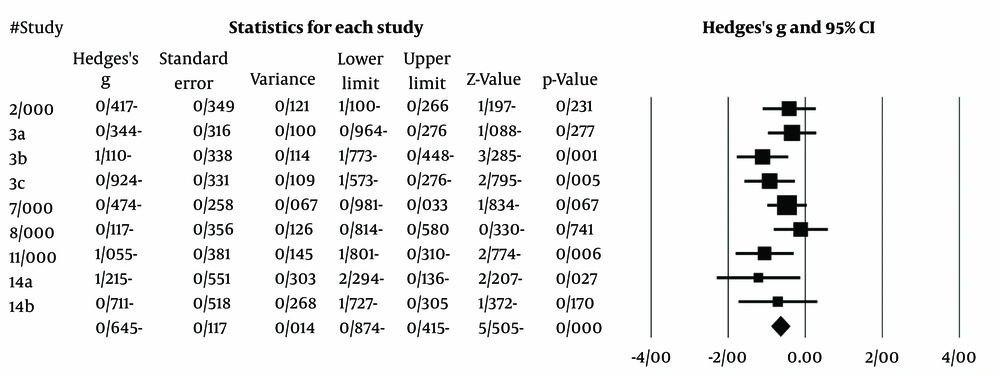
Following the removal of outlier effect sizes, the analysis was re-conducted. Regarding the effectiveness data, results from craving favored active tDCS over sham tDCS at the end of treatment (Hedges' g: -0.64; SMD -0.58, 95% CI -0.85 to -0.30; studies = 6, participants = 220; I² = 60%). The study by Ekhtiari et al. (20) has the highest weight in the meta-analysis calculation. The largest effect size is associated with the study by Hemzadeh (26). The forest plot for the meta-analysis studies is presented in Figure 7.
5. Discussion
The present systematic review and meta-analysis aimed to investigate the effectiveness of tDCS in individuals with MUD. An examination of 495 individuals with MUD revealed that tDCS can be effective in reducing momentary and cue-induced cravings. However, the overall quality of the studies was not high, and the sample sizes were generally small. Several studies included active control groups, making it difficult to isolate the effectiveness of tDCS alone versus sham control. Other important factors, such as age, clinical assessments, substance use details, duration of use, comorbidities related to MUD, and time of first use, could contribute to the heterogeneity observed in the review. Various scales were used to evaluate cravings, leading to inconsistencies, particularly in the cognitive domain. For instance, while some studies assessed memory, different scales were used to measure working memory, verbal memory, and other types of memory, with no consensus on a single instrument for assessing working memory. Furthermore, the use of neurocognitive computer assessments versus paper-and-pencil methods highlighted differences in findings within the cognitive domain. Additionally, the variability in tDCS protocols—such as stimulation site, duration, number of treatment sessions, and combination with other therapeutic methods—likely contributed to inconsistent results.
The literature review shows that individuals with MUD exhibit difficulties in executive functions, decision-making, and inhibition (2, 4, 5). These deficits are attributed to the prefrontal cortex (PFC), particularly the dorsolateral prefrontal cortex. Our review found that all studies included interventions targeting the DLPFC. Abnormal DLPFC activity has been addressed in various psychiatric conditions (27), and dopaminergic imbalances in the brain can lead to drug-taking and reward-motivated behaviors in individuals with addiction (28). Given the role of the prefrontal cortex in regulating self-control and its influence on compulsive drug-taking (29), targeting the DLPFC in tDCS protocols is important. Therefore, we recommend that the DLPFC should remain a focus in tDCS interventions aimed at improving cognitive function and modulating motivated behavior in addiction.
Another important question regarding the DLPFC is its lateralization. Some studies focus on stimulating the right DLPFC, while others target the left DLPFC. While the differentiation between the left and right DLPFC has been well-documented in language studies (30), there is evidence supporting the involvement of both sides in studies focusing on cognition (7, 15). The positive effects on addictive behaviors observed with both left- and right-sided stimulation may be due to the diffuse current flow and nonfocal effects of conventional tDCS (20). Based on our meta-analysis, studies stimulating the right DLPFC showed a greater effect size than those stimulating the left DLPFC. Furthermore, a previous meta-analysis reported that right-sided tDCS on the DLPFC can be more effective in reducing cravings than left-sided stimulation (31).
In addition to evaluating cravings and cognitive functions, some studies in our review assessed the role of emotions. It has been shown that individuals using methamphetamine often exhibit emotional dysregulation, including anxiety, depression, aggression, hostility, and irritability, especially during early abstinence (32). The PANAS was the most commonly used instrument for assessing emotional disturbances following tDCS intervention. Since tDCS does not directly support the enhancement of emotion regulation (33), improvements in emotions may be attributed to progress made during the abstinence period. Therefore, evaluating all aspects of emotions should be considered in this type of treatment.
This systematic review and meta-analysis has several limitations. As mentioned earlier, the overall quality of the studies was not high, and the sample sizes were generally small. Furthermore, cravings and cognitive functions were assessed using different scales, making it difficult to compare studies. The lack of attention to co-occurring factors related to methamphetamine use and the insufficient reporting of participants' previous experiences also limit the interpretation of the results. Additionally, the inclusion of complementary treatments alongside tDCS complicates comparisons between tDCS and control groups. Many studies did not include follow-up assessments, which are crucial in addiction treatment for evaluating relapse prevention and the long-term effectiveness of tDCS.
From our perspective, there is a need for larger RCTs and the use of standardized, less heterogeneous measurement tools. Additionally, collecting more detailed information about participants, such as clinical conditions, duration of use, and the time of initial use, will enhance data analysis. Finally, trials with diverse group characteristics, comprehensive cognitive and emotional assessments, and a greater focus on specific brain areas may improve our understanding of the application of neurostimulation in MUD.