1. Background
The phenomenon of opioid dependence and abuse has become one of the world’s major challenges, ranked as the fourth global crisis following the nuclear threat, population growth, and environmental pollution (1). Initially perceived as an individual health risk, opioid abuse has evolved into a broader socio-economic, health, and security crisis for many nations (2). The prevalence of substance abuse is rapidly increasing both globally and within Iran, with a significant portion of opioid users being adolescents and young adults (3). Over the past 20 years in Iran, the growth rate of substance abuse has been more than three times the population growth rate (4). Controlling opioid dependence is a critical issue for Iran. Given its 1,925-kilometer shared border with Afghanistan and Pakistan — two of the world’s largest opium poppy and heroin production regions — Iran lies along the transit route for opioids from these countries to Europe, rendering it particularly vulnerable (1). Treating substance abuse and breaking the vicious cycle of addiction is both costly and complex, requiring a comprehensive treatment system incorporating pharmacotherapy, psychotherapy, and rehabilitation approaches. In this context, prevention logically becomes a priority over treatment. Today, substance abuse is not only an individual and social problem but also a family issue. It adversely affects the mental health (MH) of both opioid users and their families (5).
Currently, understanding the problems associated with substance abuse and developing strategies to control and reduce opioid addiction is one of Iran’s key priorities (1). Nearly 90% of opioid users suffer from some form of mental disorder, with depression, antisocial personality disorder, and anxiety being particularly significant (6). Among anxiety disorders, phobias are most frequently associated with depression. Moreover, the decreased energy and hopelessness stemming from depression can reduce the motivation of opioid users to quit drugs and pursue treatment (1). The prevalence of depression among opioid users ranges between 50% and 60%, while minor depression affects approximately 10% of users. Additionally, the overall prevalence of substance abuse among patients with psychiatric disorders is about 29%, and among those with depression seeking treatment at psychiatric clinics, it rises to 56% (7). It is estimated that 40% of individuals with substance abuse (both opioid and non-opioid) have, at some point in their lives, met the criteria for major depressive disorder (8). Quality of life is a valid metric for evaluating the outcomes of therapeutic methods and services provided to individuals affected by substance abuse (9). Quality of life encompasses physical health, psychological status, social relationships, and spiritual and personal beliefs, and is assessed based on individuals’ subjective experiences. It is essential to evaluate the quality of life of opioid users, particularly those undergoing different treatment approaches to avoid substance use, especially opioid users undergoing methadone maintenance treatment, which is one of the primary methods for treating substance abuse in the country (10). Methadone is used as a biological treatment for detoxification and maintenance therapy in cases of opioid abuse, including heroin and other opiates. Its low cost, combined with its high effectiveness in controlling the physical and psychological conditions associated with opioid dependence, has made methadone a practical and widely used medication in this field (11).
Although methadone maintenance treatment may be viewed as a form of physical substance dependence on the drug, it is not equated with substance abuse. This is because regular use of methadone frees individuals from the cycle of euphoria, withdrawal, and compulsive substance use, enabling them to re-enter society and redirect their life energy toward other areas (12). Research findings on the impact of methadone maintenance treatment on the MH of opioid users have been inconsistent. For example, some studies have shown that compared to the general population, individuals undergoing methadone treatment exhibit a high level of MH issues, frequently experiencing mood and emotional disorders such as depression and anxiety (13). In methadone treatment programs, the drug is administered in controlled doses as an oral syrup or tablet at designated centers. Experts believe that methadone substitution reduces the prevalence of intravenous drug use and the transmission of dangerous diseases such as HIV/AIDS. Additionally, it cuts off the connection between opioid users and drug dealers, reducing the likelihood of criminal activity (1). Given that in recent years, opioid cessation programs have become increasingly widespread in Iran, the acceptance and benefits of such treatments have drawn significant attention.
Zinc is a micronutrient and a trace element, and although required in very small amounts, it is essential for human health (14). Zinc deficiency can lead to metabolic disorders and severe symptoms such as anemia, drowsiness, loss of appetite, weight loss, allergies, immune system suppression, and delayed wound healing. Long-term zinc deficiency can also result in neurobiological changes, including emotional disorders, irritability, and depression (15). Today, zinc deficiency is recognized as a nutritional problem worldwide, both in developed and developing countries. The World Health Organization (WHO) estimates that approximately one-third of the global population suffers from zinc deficiency, with around 800,000 deaths annually potentially attributable to this deficiency (16). Factors such as diets rich in fiber and phytate-containing grains (e.g., bread and rice), iron supplements, gastrointestinal diseases, smoking, and chronic stress can alter plasma zinc concentrations (17). Therefore, stress is one of the factors that reduces zinc levels (18).
Zinc, as a micronutrient, plays a vital role in various bodily functions. Dysregulation of zinc metabolism has been linked to immune dysfunction, developmental disorders, and gastrointestinal complications (19-22). The human body contains 2 - 3 mg of zinc, most of which is concentrated in the hippocampus, amygdala, and prefrontal cortex, which are involved in emotion (19). In addition, studies have shown that zinc supplementation improves mood in both antidepressant-treated and healthy individuals (22). In this study, the effects of zinc supplementation on depression in methadone-treated opioid-dependent patients were evaluated by administering zinc supplements to zinc-deficient patients. Given that substance abuse leads to anxiety and depression, and various studies have shown that stress reduces serum zinc levels, and considering the significant damage anxiety and depression cause to opioid users — threatening patients’ health and impairing productivity — it is crucial to address this issue.
2. Objectives
As there is no prior research on the effects of zinc supplementation on anxiety and depression in opioid users receiving methadone maintenance therapy (MMT), this study aims to evaluate the impact of zinc supplementation on the likelihood of relapse into anxiety and depression in opioid-dependent patients undergoing MMT at Golestan Hospital in Ahvaz. The findings are intended to contribute effectively to reducing the recurrence of anxiety and depression in these patients.
3. Patients and Methods
3.1. Patients
Seventy patients were selected using convenience sampling from those willing to participate in the study, based on inclusion and exclusion criteria. These patients were randomly assigned into two groups through a lottery system. It is important to note that in experimental research, each subgroup must include at least 15 participants to ensure that the selected sample accurately represents the population, thereby enhancing the study’s external validity (23, 24). In this study, after obtaining ethical approval (IR.AJUMS.REC.1402.119) and registering the randomized clinical trial (IRCT20160417027437N3) at Ahvaz University of Medical Sciences, the entire procedure was thoroughly explained to the patients. After outlining the stages and conditions, an informed consent form — based on the Declaration of Helsinki — was provided to the patients. They were asked to read it carefully and sign if they agreed to participate. Eligible individuals were then randomly and double-blindly selected and divided into two groups: Intervention and control.
After explaining the study to the patients and obtaining their consent, those who met the inclusion criteria were randomly assigned using a randomization list, which was based on the random block permutation method to eliminate confounding factors. The patients were equally divided into two groups: The intervention group (zinc sulfate) and the control group (placebo), with 35 patients in each group. Patients in the intervention group were administered 220 mg zinc sulfate capsules every other day, while those in the control group received a placebo under the same regimen. Patients received zinc supplements for three months. Patients were followed up for three months while taking zinc. Questionnaires were filled out before starting zinc, and then again every two weeks while taking zinc, to determine changes in anxiety and depression, which took up to three months.
3.1.1. Inclusion Criteria
Inclusion criteria consisted of: (1) Age between 18 and 60 years; (2) receiving MMT as the sole treatment protocol; (3) diagnosis of substance use disorder based on DSM-V criteria; (4) willingness to participate in the study; (5) positive opioid use confirmed by urine test, with no use of other drugs (stimulants, hallucinogens); (6) receiving methadone under medical supervision for at least three months; (7) no major neurological or psychiatric conditions (e.g., schizophrenia, bipolar disorder), and no underlying medical conditions (e.g., diabetes, rheumatism, autoimmune diseases); (8) not taking medications that reduce serum zinc levels (quinolone antibiotics, bisphosphonates like alendronate, tetracycline, doxycycline, and minocycline, antihypertensive medications such as thiazides, verapamil, etc.), and avoiding foods high in calcium (dairy), fiber (whole grains), and iron (due to competitive absorption).
3.1.2. Exclusion Criteria
Exclusion criteria included: (1) History of allergy to zinc-containing compounds; (2) use of zinc supplements or other medications before the intervention and study; (3) pregnancy and breastfeeding; (4) having thoughts of self-harm or harm to others; (5) aggression or use of psychiatric medications in the past three months; (6) discontinuation of participation for any reason; (7) discontinuation of methadone treatment during the study.
3.2. Data Collection Tools
The Beck Depression Inventory-II (BDI-II), whose validity and reliability have been evaluated in domestic studies, was used. The Cronbach’s alpha coefficient for this test was reported to be 96% in a study by Dabson and Mohammad Khani (13). The Beck test consists of 21 questions, each with four options. If a respondent selects the first option for all questions, they will score 0, while selecting the fourth option for all questions results in a score of 63. In this study, participants with scores: (1) Below 16 were considered non-depressed, (2) scores between 17 - 25 indicated mild depression, (3) scores between 26 - 33 indicated moderate depression, (4) and scores above 34 indicated severe depression.
To assess quality of life, the short-form 26-item questionnaire from the World Health Organization (WHOQOL-26) was used. This questionnaire includes 26 questions covering four areas: Physical health, psychological state, social relationships, and living conditions, which are used to reflect overall quality of life and general health levels. This questionnaire has also demonstrated favorable validity and reliability in domestic studies. In another study, the Cronbach’s alpha coefficient was calculated to be 84% (25, 26). The questions were rated on a five-point Likert scale (never, rarely, moderately, often, and very often).
To measure anxiety levels, the Spielberger Anxiety Inventory was used, which was developed by Spielberger in 1983 as a self-assessment tool with two separate forms. It consists of 40 items that allow respondents to rate their feelings, with a score of 1 indicating no anxiety and a score of 4 indicating high anxiety. Ultimately, all scores were transformed into percentages, with the lowest score set at 0 and the highest at 100. In recent years, this scale has been the most widely used tool for assessing anxiety in various studies inside and outside the country. It was reported that the reliability coefficient for this scale was calculated to be 93% for state anxiety and 90% for trait anxiety (26).
3.3. Sample Size Calculation Method
Based on previous studies and the data provided in the table below, the sample size for each group was estimated to be 31 participants. However, considering a potential 10% dropout rate, it was deemed appropriate to include 35 participants in each group (27).
N1 = 38; N2 = 38; Ratio = 1; α = 0.05; β = 0.80; Mean1 = 26.00; Mean2 = 21.38; S1 = 6.14; S2 = 6.51
3.4. Statistical Methods
Quantitative variables were reported using the mean and standard deviation. In cases where the data did not follow a normal distribution, the median and interquartile range were utilized. For reporting qualitative variables, percentage and frequency were employed. To compare the levels of anxiety and depression over time (before the intervention, one month after the intervention, and three months after the intervention), repeated measures analysis was used. Additionally, independent t-tests (or the Mann-Whitney test if the data were not normally distributed) were applied to compare outcomes between the first and third months. A significance level of 5% was considered, and the analysis was performed using SPSS version 26.
4. Results
Seventy participants were included in the study, with 35 in the intervention group and 35 in the control group. One month after the initiation of the program, three participants in the intervention group were removed due to non-adherence to medication during follow-ups, and four participants in the control group were excluded from the study due to refusal to cooperate further. Ultimately, the collected data from 63 patients with opioid use disorder were analyzed, comprising 32 individuals in the intervention group and 31 in the control group.
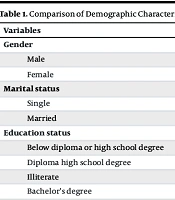
There was no significant difference in age between the two groups (P = 0.832, Cohen’s d = 9.5). Additionally, there was no significant difference in the amount of methamphetamine consumption between the intervention group (47.82 ± 122.75) and the control group (42.79 ± 127.28) (P = 0.665). There was no significant difference in gender distribution between the case and control groups (P = 0.754). There was also no significant difference in marital status distribution between the case and control groups (P = 0.367).
The chi-square test showed that there is a significant difference in the distribution of education levels between the case and control groups (P = 0.080), with a higher number of individuals having less than a high school diploma. There was no significant difference in employment status distribution between the case and control groups (P = 0.353) (Table 1).
| Variables | Case Group Number | Control Group | P-Value |
|---|---|---|---|
| Gender | 0.754 | ||
| Male | 34 (97.1) | 34 (97.1) | |
| Female | 1 (2.9) | 1 (2.9) | |
| Marital status | 0.367 | ||
| Single | 6 (17.1) | 4 (11.4) | |
| Married | 29 (82.9) | 31 (88.6) | |
| Education status | 0.080 | ||
| Below diploma or high school degree | 21 (60) | 27 (77.1) | |
| Diploma high school degree | 12 (34.3) | 4 (11.4) | |
| Illiterate | 2 (5.7) | 2 (5.7) | |
| Bachelor’s degree | 0 (0) | 2 (5.7) | |
| Employment status | 0.353 | ||
| Unemployed | 15 (42.9) | 10 (28.6) | |
| Self-employed | 16 (45.7) | 19 (54.3) | |
| Laborer | 2 (5.7) | 5 (14.3) | |
| Associate nurse assistant | 1 (2.9) | 0 (0) | |
| Butcher | 1 (2.9) | 0 (0) | |
| Employee | 0 (0) | 1 (2.9) |
Comparison of Demographic Characteristics Between the Case and Control Groups a
The t-test showed that there is a significant increase in the following variables when comparing the case and control groups: Quality of life-physical health three months after treatment, quality of life-overall physical health, quality of life-MH at the beginning of the study, quality of life- MH one month after taking zinc, quality of life-MH three months after zinc consumption, quality of life-overall MH, quality of life-overall MH one and three months after taking zinc, quality of life-social health three months after treatment, quality of life-overall social health three months after treatment, quality of life overall, and quality of life overall three months after taking zinc (P < 0.05) (Table 2).
| Quality of Life Variables | Mean ± SD | P-Value | Cohen’s d |
|---|---|---|---|
| Physical health at the beginning of the study | 0.560 | 2.8 | |
| Case | 27.45 ± 3.37 | ||
| Control | 27.05 ± 2.22 | ||
| Physical health one month after taking zinc | 0.158 | 3.01 | |
| Case | 28.50 ± 3.59 | ||
| Control | 27.41 ± 2.27 | ||
| Physical health three months after taking zinc | 0.010 | 3.19 | |
| Case | 29.96 ± 3.61 | ||
| Control | 27.83 ± 2.68 | ||
| Overall physical health at the beginning of the study | 0.582 | 3.02 | |
| Case | 2.22 ± 3.53 | ||
| Control | 1.82 ± 2.41 | ||
| Overall physical health one month after taking zinc | 0.178 | 2.97 | |
| Case | 3.43 ± 3.52 | ||
| Control | 2.41 ± 2.27 | ||
| Overall physical health three months after taking zinc | 0.010 | 3.19 | |
| Case | 4.96 ± 3.61 | ||
| Control | 2.83 ± 2.68 | ||
| MH at the beginning of the study | < 0.001 | 4.00 | |
| Case | 21.22 ± 3.82 | ||
| Control | 17.51 ± 4.18 | ||
| MH one month after taking zinc | 0.001 | 4.36 | |
| Case | 22.00 ± 4.15 | ||
| Control | 18.22 ± 4.58 | ||
| MH three months after taking zinc | < 0.001 | 4.54 | |
| Case | 24.37 ± 4.19 | ||
| Control | 18.74 ± 4.87 | ||
| Overall MH at the beginning of the study | < 0.001 | 4.00 | |
| Case | -3.77 ± 3.82 | ||
| Control | -7.48 ± 4.18 | ||
| Overall MH one month after taking zinc | 0.001 | 4.36 | |
| Case | -3.00 ± 4.15 | ||
| Control | -6.77 ± 4.58 | ||
| Overall MH three months after taking zinc | < 0.001 | 4.63 | |
| Case | -0.62 ± 4.19 | ||
| Control | -6.38 ± 5.05 | ||
| Social relationships at the start of the study | 0.576 | 1.91 | |
| Case | 9.88 ± 1.79 | ||
| Control | 9.62 ± 2.03 | ||
| Social relationships one month after taking zinc | 0.569 | 1.95 | |
| Case | 10.25 ± 1.93 | ||
| Control | 9.96 ± 1.97 | ||
| Social relationships three months after taking zinc | 0.024 | 2.06 | |
| Case | 11.43 ± 2.04 | ||
| Control | 10.22 ± 2.09 | ||
| Overall social relationships at the start of the study | 0.494 | 1.91 | |
| Case | -15.11 ± 1.79 | ||
| Control | -15.42 ± 2.01 | ||
| Overall social relationships one month after taking zinc | 0.484 | 1.95 | |
| Case | -14.75 ± 1.93 | ||
| Control | -15.09 ± 1.97 | ||
| Overall social relationships three months after taking zinc | 0.018 | 2.07 | |
| Case | -13.56 ± 2.04 | ||
| Control | -14.83 ± 2.09 | ||
| Environmental health at the start of the study | 0.581 | 5.82 | |
| Case | 23.80 ± 5.79 | ||
| Control | 24.57 ± 5.84 | ||
| Environmental health one month after taking zinc | 0.628 | 5.89 | |
| Case | 24.46 ± 5.83 | ||
| Control | 25.19 ± 5.96 | ||
| Environmental health three months after taking zinc | 0.517 | 6.14 | |
| Case | 26.71 ± 6.01 | ||
| Control | 25.70 ± 6.27 | ||
| Overall environmental health at the start of the study | 0.581 | 5.82 | |
| Case | -1.20 ± 5.79 | ||
| Control | -0.42 ± 5.84 | ||
| Overall environmental health one month after taking zinc | 0.628 | 5.89 | |
| Case | -0.53 ± 5.83 | ||
| Control | 0.19 ± 5.96 | ||
| Overall environmental health three months after taking zinc | 0.490 | 6.13 | |
| Case | 1.71 ± 6.01 | ||
| Control | 0.64 ± 6.25 | ||
| At the start of the study | 0.691 | 1.19 | |
| Case | 6.00 ± 1.16 | ||
| Control | 5.88 ± 1.23 | ||
| One month after taking zinc | 0.556 | 1.23 | |
| Case | 6.28 ± 1.19 | ||
| Control | 6.09 ± 1.27 | ||
| Three months after taking zinc | 0.022 | 1.25 | |
| Case | 7.09 ± 1.17 | ||
| Control | 6.29 ± 1.32 | ||
| Overall at the start of the study | 0.691 | 1.19 | |
| Case | -19.00 ± 1.16 | ||
| Control | -10.11 ± 1.23 | ||
| Overall one month after taking zinc | 0.556 | 1.23 | |
| Case | -18.71 ± 1.19 | ||
| Control | -18.90 ± 1.27 | ||
| Overall three months after taking zinc | 0.022 | 1.25 | |
| Case | -27.96 ± 1.17 | ||
| Control | -18.70 ± 1.32 |
Comparison of Quality of Life Scores Between Case and Control Groups
The chi-square test showed that there is a significant difference in the DASS 21 scores between the case and control groups. After three months of zinc supplementation, the levels of anxiety, stress, and depression in the case group significantly decreased compared to the control group (P = 0.038) (Table 3).
| DASS21 Score | Case Group Number | Control Group | P-Value |
|---|---|---|---|
| At the beginning of the study | 0.889 | ||
| Average-average | 15 (42.9) | 17 (48.6) | |
| Mild-mild | 19 (54.3) | 17 (48.6) | |
| Severe-severe | 1 (2.9) | 1 (2.9) | |
| One month after taking zinc | 0.116 | ||
| Average-average | 12 (44.4) | 4 (100) | |
| Mild-mild | 13 (48.1) | 0 (0) | |
| Normal-normal | 2 (4.7) | 0 (0) | |
| Three months after taking zinc | 0.038 | ||
| Average-average | 6 (18.8) | 13 (41.9) | |
| Mild-mild | 14 (43.8) | 14 (45.2) | |
| Normal-normal | 12 (37.5) | 4 (12.9) |
Comparison of DASS 21 Scores Between Case and Control Groups a
The chi-square test showed that there is a significant difference in the BDI scores between the case and control groups. After three months of zinc supplementation, the level of depression in the case group significantly decreased compared to the control group (P = 0.038) (Table 4).
| Score | Case Group | Control Group | P-Value |
|---|---|---|---|
| At the beginning of the study | 0.889 | ||
| Average | 15 (42.9) | 17 (48.6) | |
| Mild | 19 (54.3) | 17 (48.6) | |
| Severe | 1 (2.9) | 1 (2.9) | |
| One month after taking zinc | 0.572 | ||
| Average | 12 (37.5) | 15 (48.4) | |
| Mild | 16 (50) | 14 (45.2) | |
| Normal | 4 (12.5) | 2 (6.5) | |
| Three months after taking zinc | 0.038 | ||
| Average | 6 (18.8) | 13 (41.9) | |
| Mild | 14 (43.8) | 14 (45.2) | |
| Normal | 12 (37.5) | 4 (12.9) |
Comparison of Beck Depression Inventory-II Scores Between the Case and Control Groups a
As depicted by Table 5, no significant difference existed in the Spielberger Anxiety Inventory scores between the case and control groups (P > 0.05).
| Spielberger Score | Case Group | Control Group | P-Value |
|---|---|---|---|
| At the beginning of the study | 0.889 | ||
| Average to high | 1 (2.9) | 4 (11.4) | |
| Average to low | 14 (40) | 13 (37.1) | |
| Mild | 19 (54.3) | 17 (48.6) | |
| Relatively severe | 1 (2.9) | 0 (0) | |
| Severe | 0 (0) | 1 (2.9) | |
| One month after taking zinc | 0.671 | ||
| Average to high | 2 (6.3) | 4 (12.9) | |
| Average to low | 9 (28.1) | 11 (35.5) | |
| Mild | 18 (56.3) | 14 (45.2) | |
| Normal | 3 (9.4) | 2 (6.5) | |
| Three months after taking zinc | 0.074 | ||
| Average to high | 1 (3.1) | 4 (12.9) | |
| Average to low | 5 (15.6) | 9 (29) | |
| Mild | 14 (43.8) | 14 (45.2) | |
| Normal | 12 (37.5) | 4 (12.9) |
Comparison of Spielberger Anxiety Inventory Scores Between the Case and Control Groups a
All patients in the case and control groups tested positive for methadone at the beginning of the study, and at two, four, six, eight, ten, and twelve weeks after the start of the study. According to the above table, two individuals in the case group and three individuals in the control group tested positive for THC at the beginning of the study and two and four weeks after the study; one individual from the control group tested positive for THC at eight weeks after the study, while the rest tested negative.
The chi-square test showed that three individuals in the case group and seven individuals in the control group tested positive for morphine at the beginning of the study and two weeks after the study; two individuals in the case group and five individuals in the control group tested positive for morphine at four weeks after the study; one individual in the case group and four individuals in the control group tested positive for morphine at six weeks after the study; and one individual in the control group tested positive for morphine at eight weeks after the study.
Two individuals in the case group and three individuals in the control group tested positive for amphetamine at the beginning of the study and two weeks after the study; one individual in the case group and three individuals in the control group tested positive for amphetamine at four and six weeks after the study; and two individuals in the control group tested positive for amphetamine at eight weeks after the study (Table 6).
| Test’s Result | Case | Control | P-Value |
|---|---|---|---|
| THC | |||
| THC + at the beginning of the study | 2 (5.9) | 3 (8.6) | 0.514 |
| THC - at the beginning of the study | 32 (94.1) | 32 (91.4) | 0.514 |
| THC + two weeks after taking zinc | 2 (5.9) | 3 (8.6) | 0.514 |
| THC - two weeks after taking zinc | 32 (94.1) | 32 (91.4) | 0.514 |
| THC + four weeks after taking zinc | 2 (6.3) | 3 (9.7) | 0.485 |
| THC - four weeks after taking zinc | 30 (93.8) | 28 (90.3) | 0.485 |
| THC + six weeks after taking zinc | 0 (0) | 1 (3.2) | 0.492 |
| THC - six weeks after taking zinc | 32 (100) | 30 (96.8) | 0.492 |
| THC + eight weeks after taking zinc | 0 (0) | 1 (3.2) | 0.492 |
| THC - eight weeks after taking zinc | 32 (100) | 30 (96.8) | 0.492 |
| THC + 10 weeks after taking zinc | 0 (0) | 0 (0) | - |
| THC - 10 weeks after taking zinc | 32 (100) | 31 (100) | - |
| THC + 12 weeks after taking zinc | 0 (0) | 0 (0) | - |
| THC - 12 weeks after taking zinc | 32 (100) | 31 (100) | - |
| Morphine | |||
| Morphine - at the beginning of the study | 32 (91.4) | 28 (80) | 0.153 |
| Morphine + at the beginning of the study | 3 (8.6) | 7 (20) | 0.153 |
| Morphine - two weeks after taking zinc | 32 (91.4) | 28 (28) | 0.153 |
| Morphine + two weeks after taking zinc | 3 (8.6) | 7 (20) | 0.153 |
| Morphine - four weeks after taking zinc | 30 (93.8) | 26 (83.9) | 0.200 |
| Morphine + four weeks after taking zinc | 2 (6.3) | 5 (16.1) | 0.200 |
| Morphine - six weeks after taking zinc | 31 (96.9) | 27 (87.1) | 0.167 |
| Morphine + six weeks after taking zinc | 1 (3.1) | 4 (12.9) | 0.167 |
| Morphine - eight weeks after taking zinc | 32 (100) | 31 (96.9) | 0.500 |
| Morphine + eight weeks after taking zinc | 0 (0) | 1 (3.1) | 0.500 |
| Morphine - 10 weeks after taking zinc | 32 (100) | 31 (100) | - |
| Morphine + 10 weeks after taking zinc | 0 (0) | 0 (0) | - |
| Morphine - 12 weeks after taking zinc | 32 (100) | 31 (100) | - |
| Morphine + 12 weeks after taking zinc | 0 (0) | 0 (0) | - |
| Amphetamine | |||
| Amphetamine - at the beginning of the study | 33 (94.3) | 32 (91.4) | 0.500 |
| Amphetamine + at the beginning of the study | 2 (5.7) | 3 (8.6) | 0.500 |
| Amphetamine - two weeks after taking zinc | 33 (94.3) | 32 (91.4) | 0.500 |
| Amphetamine + two weeks after taking zinc | 2 (5.7) | 3 (8.6) | 0.500 |
| Amphetamine - four weeks after taking zinc | 31 (96.9) | 28 (90.3) | 0.294 |
| Amphetamine + four weeks after taking zinc | 1 (3.1) | 3 (9.7) | 0.294 |
| Amphetamine - six weeks after taking zinc | 31 (96.9) | 28 (90.3) | 0.294 |
| Amphetamine + six weeks after taking zinc | 1 (3.1) | 3 (9.7) | 0.294 |
| Amphetamine - eight weeks after taking zinc | 32 (100) | 29 (93.5) | 0.238 |
| Amphetamine + eight weeks after taking zinc | 0 (0) | 2 (6.5) | 0.238 |
| Amphetamine - 10 weeks after taking zinc | 32 (100) | 31 (100) | - |
| Amphetamine + 10 weeks after taking zinc | 0 (0) | 0 (0) | - |
| Amphetamine - 12 weeks after taking zinc | 32 (100) | 31 (100) | - |
| Amphetamine + 12 weeks after taking zinc | 0 (0) | 0 (0) | - |
Comparison of the Results of the Drug Addiction Test Between the Case and Control Groups
5. Discussion
The results of this study indicate that, in the case group, there was a significant increase in quality of life regarding physical health, MH, and social health after zinc consumption compared to the control group (P < 0.05). There was a significant difference in the DASS21 scores between the case and control groups, with a notable reduction in anxiety, stress, and depression in the case group three months after zinc supplementation compared to the control group (P = 0.038). Additionally, there was a significant difference in the BDI scores between the two groups, with a marked decrease in depression levels in the case group compared to the control group three months after zinc supplementation (P = 0.038). However, there was no significant difference in the Spielberger scores between the case and control groups (P > 0.05). Zinc strengthens the immune system by activating the NFKβ pathway and helps activate neuroprotectants (28).
In a study by Richert et al. in 2020, the analysis focused on self-reported MH issues among young individuals receiving outpatient treatment for substance use problems in Sweden (27). The multivariate logistic regression analysis revealed a significant association between the severity of opioid use problems and anxiety, concentration issues, aggression, hallucinations, and psychological stress stemming from trauma experiences. The conclusion highlighted the diverse treatment needs within this group of substance-using youths. Since girls report higher levels of all MH problems and a greater burden of psychosocial risk factors compared to boys, they may require more comprehensive therapeutic interventions. The relationship between more severe opioid issues and MH underscores the importance of examining this correlation in treatment contexts. A multidisciplinary approach, where co-occurring problems can be addressed simultaneously, may be the most effective treatment modality for many young people with opioid-related issues (27). In this study, high levels of stress, anxiety, and depression were also noted among opioid users.
Ranjbar et al. in 2013 investigated the effects of zinc supplementation in the treatment of depression. This study was a randomized, double-blind clinical trial involving 44 patients diagnosed with major depressive disorder, who were randomly assigned to receive either zinc supplementation or a placebo. The patients in the zinc group received 25 mg of zinc supplementation daily along with selective serotonin reuptake inhibitors (SSRIs), while the placebo group received a placebo with the same antidepressant medication for twelve weeks. The severity of depression was assessed at baseline using the BDI and was repeated at weeks six and twelve. Repeated measures analysis of variance was utilized to compare and track changes throughout the study (29).
The average Beck score in the zinc supplementation group significantly decreased by the end of week six (P < 0.01) and week twelve (P < 0.001) compared to baseline. Moreover, the average BDI score in the zinc group was significantly lower than that in the placebo group at the end of week twelve (P < 0.05). The results of this study indicate that zinc supplementation, when combined with SSRIs, significantly improves major depressive disorders more effectively than placebo combined with SSRIs (29).
Additionally, the results of this study also showed that there was a significant difference in DASS21 scores between the case and control groups, with a notable reduction in anxiety, stress, and depression in the case group three months after zinc supplementation compared to the control group (P = 0.038). Furthermore, there was a significant difference in BDI scores between the two groups, with a significant decrease in depression levels in the case group compared to the control group after three months of zinc supplementation (P = 0.038). However, no significant difference was found in the Spielberger scores between the case and control groups (P > 0.05).
Amini and Heidari Farsani, in 2023 investigated the effect of zinc supplementation on the probability of relapse (PoR) and MH issues in patients with opioid use disorder undergoing MMT. The findings indicated that compared to the control group, the likelihood of drug use (P = 0.01), craving for drugs (P = 0.002), and total RPS score (P = 0.002) were significantly lower in the intervention group. Additionally, the results showed a significant reduction in depression (P = 0.01), anxiety (P = 0.001), stress (P = 0.001), and total DASS-21 score (P = 0.001) within the intervention group. The total DASS-21 score was significantly lower (P = 0.001) in the intervention group compared to the control group. It was concluded that zinc supplementation may reduce the PoR and improve MH issues in patients with opioid use disorder undergoing MMT (30).
The results of this study also demonstrated a significant difference in DASS21 scores between the case and control groups, with a notable reduction in anxiety, stress, and depression in the case group three months after zinc supplementation compared to the control group (P = 0.038). Furthermore, there was a significant difference in BDI scores between the two groups, indicating a significant decrease in depression levels in the case group after three months of zinc supplementation compared to the control group (P = 0.038). However, no significant difference was found in the Spielberger scores between the case and control groups (P > 0.05).
Ciubotariu et al. in 2015 focused on the interaction of zinc ions with opioid dependence/addiction and analgesia, discussing whether zinc supplementation should be recommended for drug users to reduce the risk of addiction. The results from both human and animal studies indicated a decrease in serum zinc levels under opioid administration conditions, primarily attributed to increased urinary excretion (in humans) or redistribution (in animals). Additionally, animal studies showed reduced brain zinc levels in morphine-treated animals, accompanied by elevated hepatic zinc levels. There was also an increase in endogenous opioid system activity and a potential decrease in morphine excretion due to zinc. Laboratory studies demonstrated the reduced binding of opioid ligands to receptors by zinc. However, very few in vivo studies on animals regarding opioid analgesia showed controversial results, as zinc exhibited clear analgesic effects but did not enhance the analgesic effects related to opioids (31).
Yosaee et al. investigated the effects of zinc, vitamin D, and their concomitant supplementation compared with placebo on changes in BDI score, serum cortisol levels, and brain-derived neurotrophic factor (BDNF) in obese/overweight patients with depressive symptoms. This 2 × 2 factorial, double-blind, randomized, placebo-controlled trial was conducted in obese/overweight patients with depressive symptoms at the Endocrine and Metabolism Research Center (CEMR), Valiasr Hospital, Imam Khomeini between June 2016 and February 2017. The intervention period was 12 weeks. A total of 140 participants were randomly assigned to be obese or overweight (mean ± SD, 38.35 ± 6.70 years of age; mean ± SD, BMI, 30.1 ± 3.78 kg/m2) with a BMI ≥ 10. Participants were randomly assigned in a 1:1:1:1 ratio to one of four groups: 2000 IU/day vitamin D + zinc placebo; 30 mg/day zinc gluconate + vitamin D placebo; 2000 IU/day vitamin D + 30 mg/day zinc gluconate; or vitamin D placebo + zinc placebo for 12 weeks. The study showed a significant reduction in BDI-II scores among those receiving zinc, vitamin D, or a combination zinc-vitamin D supplement compared with the placebo group (P < 0.001). Zinc was significantly more effective than vitamin D in reducing depression scores. Supplementation with zinc, vitamin D, or the combination of the two had no significant effect on serum cortisol (P = 0.974) or BDNF (P = 0.076). Fifteen patients withdrew due to pregnancy (n = 1), severe anemia (n = 1), and unspecified unwillingness to continue (n = 13). Supplementation with zinc, vitamin D, or a combination of them for 12 weeks had significant beneficial effects on BDI-II scores in obese or overweight patients with a BDI-II ≥ 10 (32).
It was concluded that dietary zinc supplementation should be considered for patients undergoing opioid treatment for chronic pain associated with cancer, given the high prevalence of zinc deficiency, which has also been well-documented in drug users. The low toxicity of oral zinc further supports this idea. The main opposition to administering zinc in individuals receiving opioid treatment relates to how zinc affects opioid-induced analgesia.
5.1. Conclusions
Zinc supplementation has significantly contributed to the reduction of anxiety and depression symptoms in patients receiving MMT.
5.2. Limitations
The limited number of patients did not allow for subgroup analysis based on the type of placebo plus antidepressant drugs.
5.3. Recommendations
We suggest evaluating the effects of different doses of zinc on depression in future studies.