1. Background
Diabetes mellitus is one of the most prevalent chronic diseases worldwide, and it is currently on the increase in all countries (1). Specifically, the 1985 rate of 30 millions people with diabetes increased to 135 millions in 1995 and reached 173 millions in 2002. According to a WHO forecast, the diabetes rate will have risen to 366 millions worldwide by 2030 (2). According to the international diabetes federation, there were approximately 4.5 million cases of diabetes in Iran in 2014, and 38,079 deaths from the disease were reported that year (3).
In mammalian species, physiological functions are facilitated by plasma levels of glucose up to 100 mg/dL (4). In general, diabetes can be divided into two types: type 1 diabetes, where people have problems producing insulin due to the destruction of the cell that produces this hormone (lack of insulin) (5) and type 2 diabetes mellitus (T2DM), which accounts for nearly 90% of diabetes cases worldwide (2) and is caused by insufficient production of insulin or by a malfunction of this hormone or its receptor (resistance to insulin) (5). If patients with type 2 diabetes do not control the condition, they may experience recurrent complications of diabetes, such as renal disorders (nephropathy), eye disorders (retinopathy), and nervous system problems (neuropathy) (5). These conditions can cause serious complications, including renal failure, foot injuries, amputation, and increased risk of cardiovascular diseases (6). The risk factors that give rise to the progression of T2D are mainly an increasing body mass index (BMI), aging (7), physical inactivity, stress (8), and obesity (5).
Recently, T2DM has also been recognized as an inflammatory disease in which cytokines play an important role (9). Interleukin 6 (IL6) is a proinflammatory mediator cytokine biosynthesized by T lymphocytes, macrophages, adipocytes (10), and other sources such as endothelial cells, fibroblasts, and skeletal muscle (11). IL6 is responsible for various tasks such as controlling the activation and differentiation of T lymphocyte responses and proinflammatory responses. It also plays a role in the pathogenesis of autoimmune and inflammatory diseases (12), in the regulation of body weight, and in lipid metabolism (11).
To initiate an inflammatory response, this cytokine must bind with its receptor complex, including the Interleukin 6 receptor (IL6R) and two molecules of glycoprotein 130 (gp130) (13); the latter plays a co-receptor role (12). IL6R can occur in two forms, the first as a dimer in plasma membrane and the second in soluble form (14). Glycoprotein 130 is present in most cells, while IL6R presents in special cells like macrophages, hepatocytes, epithelial cells (10), and neutrophils (15).
Cells only have gp130 on their surfaces; for interactions with IL6, they use soluble Interleukin receptor 6 (sIL6R) (13). Generally, sIL6R is present in plasma interactions with IL6. This complex can bind to gp130 on the surface of cells without IL6R, leading to the dimerization of gp130. The above process results in signaling into cells (trans-signaling) and the creation of an inflammatory state (16). The IL6R gene is located on the chromosome 1q21 (17) and includes 10 exons and nine introns. The length of this gene is 61kb, while the length of the mRNA transcripts from this gene is 3.3kb (14).
There is little data regarding the role of IL6R polymorphisms in T2D risk.
2. Objectives
In this study, we tried to evaluate the possible correlation between IL6R gene polymorphisms and the risk of T2D in a sample of the Iranian population.
3. Patients and Methods
The participants of the study were critically selected based on levels of blood glucose after an overnight stay (fasting blood sugar). Those with levels below 100 mg/dL were placed in the healthy control group, while those with levels of 126 mg/dL were diagnosed with diabetes and placed in the case group (4). Thus, 250 outpatients with a fasting blood sugar of more than 126 mg/dL, HbA1C > 6.7%, and confirmed to have diabetes by a physician at the diabetes center in Zahedan were enrolled for the study. The same number of healthy controls with normal fasting blood glucose and without a family history of diabetes or specific systemic disorders were selected from the Razmjoo reference laboratory in Zahedan and enrolled for the research.
A peripheral blood sample was taken from both the patient and control groups in ethylenediamine tetraacetic acid (EDTA) tubes and the tubes were stored at -20°C until extraction. To prepare the serum, tubes without anticoagulant were used to measure the lipid profiles.
All samples for the controls and subjects were extracted for genomic DNA testing using the salting-out method. The quality and quantity of the extracted DNA were confirmed using various methods such as electrophoresis, NanoDrop, and a spectrophotometer (WPA, UK) (based on a ratio of 260/280).
Demographic and clinical data were recorded for the subjects and healthy controls, including the following items: age, gender, body mass index (BMI), fasting blood sugar (FBS), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). In relation to BMI, the normal range is between 18.5 and 24.9. There are three grades in terms of overweight: grade one overweight is between 25 and 29.9; grades two and three are between 30 and 39.9, and more than 40, respectively.
3.1. Genotyping
In the IL6R (rs2229238), C/T (NC 000014, GI 568815584), and (rs4845625), C/T (NC 000023, GI 568815575) were detected by means of an allele-specific primer-polymerase chain reaction (ASP-PCR).
The final volume of PCR amplification was 20 μL, which comprised 10 μL of master mix (0.2 units/μL ampliqonTaq DNA polymerase, ampliqonTaq 2x master mix, Denmark), 1 μL (10 pmol/mL) of reverse (R) and forward (F) primers, ~80 - 100 ng of template DNA and 6.5 μL of DNase-free water.
For the single nucleotide polymorphism (SNP) rs2229238, the amplification and cycling conditions were as follows: an initial denaturation of 95°C for five minutes and subsequently, repeated 30-second cycles under the following conditions: denaturation at 95°C for 30 seconds, annealing at 62°C for 30 seconds, and extension at 72°C for 25 seconds with a final extension at 72°C for five minutes.
For rs4845625, amplification was performed with an initial denaturation of 95°C for five minutes and subsequently, repeated 30-second cycles under the following conditions: denaturation at 95°C for 30 seconds, annealing at 53°C for 30 seconds, and extension at 68°C for 25 seconds with a final extension at 68°C for five minutes. The PCR products were purified on a 1.5% agarose gel containing 0.6 μg/mL ethidium bromide, and observed under UV light.
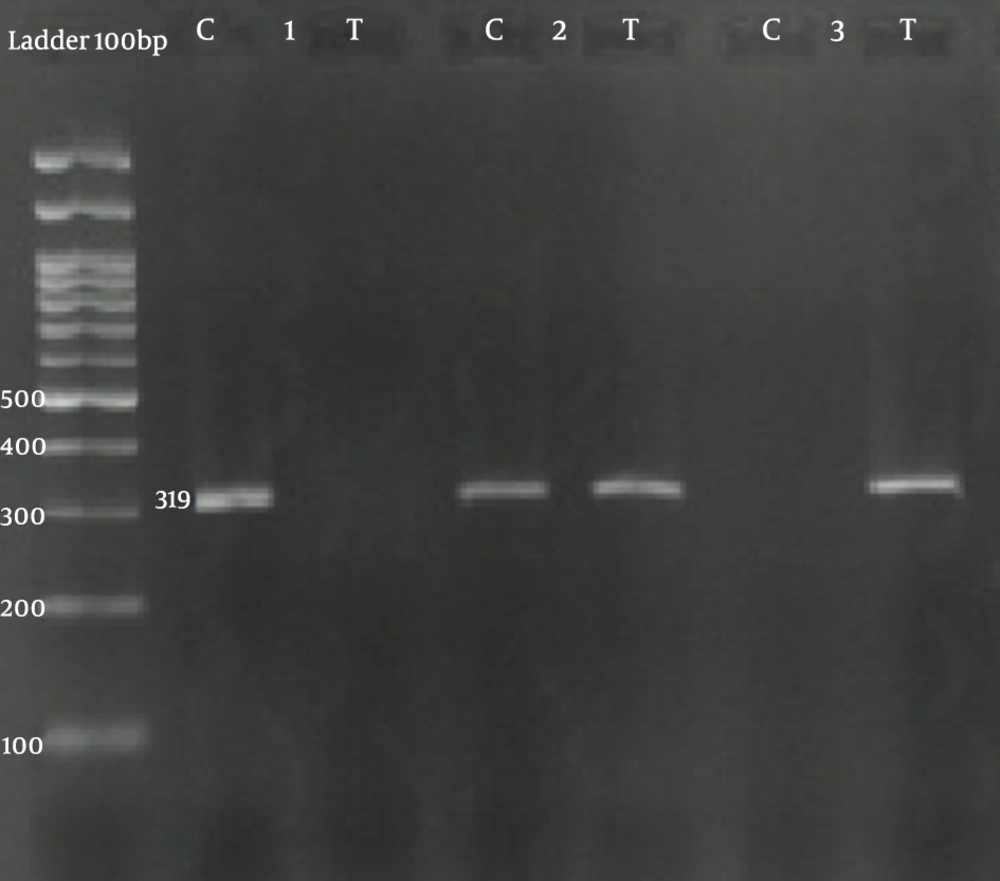
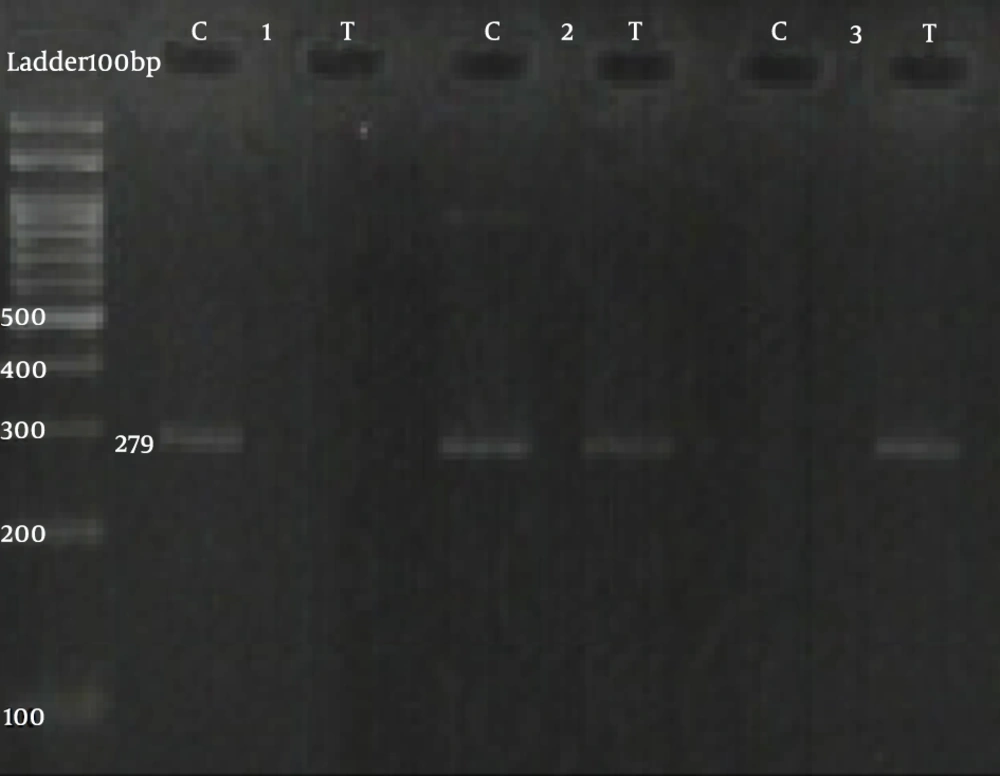
Information on SNP primers is presented in Table 1. The PCRs for both SNPs, namely, rs2229238 C/T and rs4845625 C/T, were performed using two sequence-specific forward primers (F1 and F2) and a common reverse primer (R) through the creation of a 319 bp band and a 279 bp band, respectively (Figures 1 and 2).
| Primer | Sequences (5’-3’) | Product | Method |
|---|---|---|---|
| rs2229238 | 319 bp | ARMS | |
| Fw | CCTGGACCCTGTGGATGTC | ||
| Fm | CCTGGACCCTGTGGATGTT | ||
| R | AGCAGCTTCTCCACACCGA | ||
| rs4845625 | 279 bp | ARMS | |
| Fw | GGAACCAGCATACCAGTCTC | ||
| Fm | GGAACCAGCATACCAGTCTT | ||
| R | AGTTCTGGAGCTACCTCCTC |
3.2. Statistical Analysis
SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data, and the demographic and clinical data were analyzed using the independent sample t-test and χ2 test.
The association of the rs2229238 and the rs4845625 with T2D was assessed by computing the odds ratio (OR) and 95% confidence intervals (95% CI) from the logistical regression analysis. In the case of all the tests, P ≤ 0.05 was considered to be significant.
4. Results
The study was performed on 250 Iranian diabetic patients (183 females and 67 males) with a mean age of 54.87 ± 10.13 and the same number of healthy subjects (174 females and 76 males) with an average age of 48.85 ± 10.01. The demographic characteristics of the T2D patients and the controls are summarized in Table 2.
| Characteristics | T2D (n = 250) | Controls | P-Value | ||
|---|---|---|---|---|---|
| mean ± SD | N | mean ± SD | N | ||
| Age | 54.87 ± 10.13 | 48.85±10.01 | < 0.0001 | ||
| Sex | NS | ||||
| Female | 183 | 174 | |||
| Male | 67 | 76 | |||
| FBS | 188.40 ± 86.45 | 97.70 ± 19.21 | < 0.0001 | ||
| TC | 183 ± 44.31 | 181.84 ± 36.1 | NS | ||
| TG | 161.40 ± 83.24 | 148.1 ± 94.59 | NS | ||
| HDL | 55.4 ± 20.17 | 54.01 ± 14.68 | NS | ||
| LDL | 97.21 ± 34.33 | 104.49 ± 29.06 | 0.002 | ||
| BMI | 27.63 ± 5.49 | 21.48 ± 2.43 | < 0.0001 | ||
Abbreviation: NS, not significant.
4.1. Genotyping of ILR6 (rs2229238)
In the T2D group, the genotype frequencies in the rs2229238 polymorphism of the IL6R gene for CC, CT, and TT were 89.2%, 9.6%, and 1.2%, respectively, and in the control group, they were 73.6%, 20.8%, and 5.6%, respectively. In the evaluation of allele frequencies for the rs2229238, it was found that the C allele frequency was 94% and 84% in the T2D and control groups, respectively. This result showed that the CT genotype (P = 0.000) and the TT genotype (P = 0.007) in the subject group and control group were significantly different, and that this SNP plays a protective role against T2D. By evaluating the allele, we found a significant difference between the subject and control groups. The T allele (P = 0.000) suggested that this SNP plays a protective role against T2D.
4.2. Genotyping of ILR6 (rs4845625)
In the T2D group, the genotype frequencies in the rs4845625 polymorphism of the IL6R gene for CC, CT, and TT were 28.8%, 20.8%, and 50.4%, respectively, and in the control group, they were 40.4%, 15.2%, and 44.4%, respectively. In the evaluation of the allele frequencies for rs4845625, it was found that the C allele frequency was 39.2% and 48% in the T2D and control groups, respectively. For the CT genotype (OR = 1.92, %95 CI = 1.15 - 3.23, P = 0.031) and the TT genotype (OR = 1.59, %95 CI = 1.08 - 2.38, P = 0.021), there was a significant difference between the groups, indicating that this polymorphism is a risk factor for T2D. The T allele (OR = 1.43, %95CI = 1.11 - 1.84, P = 0.006) was also significantly higher in the T2D group. The frequencies of the allele/genotype of IL6R, including the rs2229238 C/T and the rs4845625 C/T, are shown in Table 3 for both groups. Our findings show a very significant association between the IL6R polymorphisms and T2D in our study population.
| IL6R Polymorphisms | Type 2 Diabetes | Control | OR (95% CI) | P Value |
|---|---|---|---|---|
| rs2229238 | ||||
| CC | 223 (89.2) | 184 (73.6) | 1.00 | - |
| CT | 24 (9.6) | 52 (20.8) | 0.38 (0.23 - 0.65) | 0.000 |
| TT | 3 (1.2) | 14 (5.6) | 0.18 (0.05 - 0.63) | 0.007 |
| CT+TT | 27 (10.8) | 66 (26.4) | 0.34 (0.21 - 0.55) | 0.000 |
| C | 470 (94) | 420 (84) | 1.00 | |
| T | 30 (6) | 80 (16) | 0.34 (0.22 - 0.52) | 0.000 |
| rs4845625 | ||||
| CC | 72 (28.8) | 101 (40.4) | 1.00 | - |
| CT | 52 (20.8) | 38 (15.2) | 1.92 (1.15 - 3.23) | 0.031 |
| TT | 126 (50.4) | 111 (44.4) | 1.59 (1.08 - 2.38) | 0.021 |
| CT+TT | 178 (71.2) | 149 (59.6) | 1.68 (1.15 - 2.43) | 0.007 |
| C | 196 (39.2) | 240 (48) | 1.00 | |
| T | 304 (60.8) | 260 (52) | 1.43 (1.11 - 1.84) | 0.006 |
aValues are expressed as No. (%).
The statistical analysis presented in Table 4 regarding the demographic and clinical data demonstrates that there was no significant association between the CC genotype and the CT+TT genotype in the subject group with the exception of BMI (P = 0.023) for the rs4845625 polymorphism and HDL (P = 0.025) in the control group for the rs2229238 SNP.
| Genotype | Age, y | Sex (male/female) | BMI, Kg/m2 | TC, mg/dL | TG, mg/dL | HDL, mg/dL | LDL, mg/dL |
|---|---|---|---|---|---|---|---|
| T2D Patients | |||||||
| rs2229238 | |||||||
| CC | 54.97 ± 10.09 | 61(M)/162(F) | 27.66 ± 5.53 | 183.58 ± 44.93 | 161.62 ± 85.35 | 55.8 ± 18.45 | 97.23 ± 34.03 |
| CT+TT | 54.00 ± 10.56 | 6(M)/21(F) | 28.21 ± 4.29 | 180.07 ± 38.08 | 254.52 ± 62-47 | 56.75 ± 15.13 | 91.44 ± 28.45 |
| P-Value | 0.95 | 0.57 | 0.413 | 0.804 | 0.636 | 0.423 | 0.496 |
| rs4845625 | |||||||
| CC | 56.26 ± 11.31 | 22(M)/50(F) | 28.05 ± 7.6 | 185.57 ± 39.21 | 152.75 ± 76.83 | 58.58 ± 21.00 | 95.39 ± 29.8 |
| CT+TT | 54.3 ± 9.58 | 45(M)/133(F) | 27.58 ± 4.27 | 182.25 ± 46.11 | 164.13 ± 85.51 | 54.82 ± 16.72 | 97.09 ± 34.94 |
| P-Value | 0.5 | 0.394 | 0.023 | 0.259 | 0.450 | 0.260 | 0.092 |
| Control Group | |||||||
| rs2229238 | |||||||
| CC | 48.57 ± 9.86 | 60(M)/124(F) | 22.24 ± 3.12 | 179.11 ± 33.24 | 145.27 ± 86.87 | 53.1 ± 13.33 | 103.37 ± 28.49 |
| CT+TT | 49.63 ± 10.44 | 16(M)/50(F) | 22.29 ± 2.49 | 189.35 ± 42.35 | 155.94 ± 113.9 | 56.95 ± 18.25 | 107.26 ± 31.10 |
| P-Value | 0.516 | 0.205 | 0.537 | 0.054 | 0.174 | 0.025 | 0.541 |
| rs4845625 | |||||||
| CC | 48.94 ± 10.46 | 30(M)/71(F) | 23.56 ± 2.63 | 178.15 ± 32.23 | 151.16 ± 100.12 | 54.21 ± 14.50 | 104.36 ± 28.55 |
| CT+TT | 48.79 ± 9.73 | 46(M)/103(F) | 24.79 ± 2.11 | 184.46 ± 36.60 | 145.88 ± 90.76 | 53.87 ± 14.87 | 104.58 ± 29.54 |
| P-Value | 0.905 | 0.844 | 0.424 | 0.182 | 0.584 | 0.266 | 0.396 |
aData are presented as mean ± SD.
5. Discussion
In this study, we investigated the possible association of the IL6R rs2229238 and rs4845625 polymorphisms with susceptibility to T2D, as well as the relationship between BMI and T2D in a sample of the Iranian population. The results showed a significant association between the rs22229238 and the rs4845625 polymorphisms and T2D, indicating that the CT+TT genotypes of IL6R (rs2229238) decrease the risk of T2D in our population by playing a protective role. In contrast, the CT+TT genotypes of IL6R (rs4845625) increase the risk of T2D. We also analyzed the association between both SNPs (rs2229238 and rs4845625) of IL6R and the demographic and clinical data; the findings showed no significant association between these variants and T2D with the exception of BMI in the case of the rs4845625 C/T polymorphism in the T2D group and HDL in the case of the rs2229238 C/T polymorphism in the healthy controls.
There is little data regarding the role of IL6R polymorphisms in T2D risk. Qi et al. found, on examination, that the rs2229238 of the IL6 polymorphism may interact with C-reactive protein (CRP) in women and predict a diabetes risk (18). Conversely, the same researchers investigated this SNP in European Caucasian women and found no significant risk of T2D (19).
Wolford et al. investigated the IL6R rs2229238 polymorphism in Pima Indians, recognizing the fact that rs2229238 has no significant association with sex in this population (13). Horibe et al. investigated the IL6R rs4845625 polymorphism in a Japanese study sample and found that it was significantly associated with chronic kidney disease (CKD) in this population (20).
Body mass index (BMI) is measured by dividing weight (in kilograms) by the square of the height (in meters) (21). We found an association between some variants of IL6R, such as rs4845617, and BMI and obesity in a study of a sample of Spanish women (22), although the subjects with Ala358 had a lower BMI than the homozygous ASP358 alleles in this sample of the Spanish population (23). In another study, Lin et al. investigated the IL6R rs2229238 C/T polymorphism in school children in Taiwan and found that, compared to the CC genotype, girls with the T allele had a larger waist circumference (WC) and a higher waist circumference to height ratio (WHtR) (24).
The lipid profile, consisting of very low-density lipoproteins (VLDL), low-density lipoproteins (LDL), high-density lipoproteins (HDL), total cholesterol (TC), and triglycerides (TG), was found to have a proven association with inflammatory disorders such as coronary heart disease (CHD) (25) and psoriasis (26). Diabetes is determined by an increase in fasting blood sugar (FBS) with biochemical alterations in the lipid profile (27). In a previous research study, Abe et al. examined the IL6R rs4845625 polymorphism in Japanese subjects and found that rs4845625 increased the risk of hypertriglyceridemia (28). Similarly, in scrutinizing this SNP, Chu et al. found that the rs2229238 C/T variant had a strong association with high TG (16).
In our results, no significant difference was identified between the control and case groups in terms of TG, although for HDL, there was a noteworthy difference between the genotypes in the control group. In the case of BMI, significant differences between the genotypes only arose in the T2D group, which may have been due to food culture and diet in our study population. However, the current study had some limitations, such as different ethnic groups and varying economic, dietary, and environmental conditions.
Various studies have shown that substituted nucleotides (or SNPs) in the IL6R gene cause an increase in the concentration of circulating IL6; in particular, it may lead to higher quantities of adipose tissue (24, 29).
In conclusion, our findings showed that the IL6R polymorphism rs2229238 is associated with T2D, and possibly plays a protective role. On the other hand, rs4845625 is a risk factor for T2D. Conversely, in a comparison of the BMI values for the rs4845625 genotypes in the patient group and HDL values for the rs2229238 genotypes in the control group, there was a significant difference between the CC and CT+TT genotypes.