1. Background
Chronic kidney disease is a general term for heterogeneous disorders affecting kidney structure and function in blood filtration and hormone secretion. This disease is rare before the age of 45, and its prevalence increases with age, especially after 65 (1-3). In France, it is linked in almost a quarter of reported cases with hypertension and in another quarter to diabetes (4). According to a 2003 study, ten years after the onset of diabetes, one-third of patients develop kidney failure, 6% of which are in advanced stages (5), but proper management of diabetes could alter these results (4).
Glomerulonephritis is due to frequent urinary tract infections, especially in women, and the rise of the germ in the kidneys (4, 6). It was the leading cause of kidney failure in the 1990s, although this is no longer a concern since it is now the leading cause in only 12% of patients (4). More than 6% of cases are related to an inherited genetic condition called polycystic kidney disease, characterized by numerous cysts in the kidneys (4, 7). Other risk factors that may cause chronic renal disease include obesity, hyperlipidemia, smoking, urinary tract obstruction (as in prostate hypertrophy and kidney stones), and autoimmune diseases, such as systemic lupus erythematous (6-15).
Recent studies have shown that the prevalence of CKD is influenced by many factors including sex (11, 16), sleep disorders (17), amount of water consumed per day (18-20), consumption of a high protein diet (21), and excessive consumption of sodium (22, 23). Obviously, the causes of CKD vary among populations and even within the same population, according to genetic and environmental factors. Kabir et al. (11) showed that glomerulonephritis was the main cause of CKD in Bangladesh (67.2%), followed by diabetes (24%) and hypertension (4.8%). In another study in the Congo, Sumaili et al. (13) reported that the main causes of CKD were hypertension (26.6%), diabetes (11.7%), and obesity (14.9%).
The major complications of CKD are cardiovascular events, sodium accumulation in the body, and the increased secretion of hypertensive hormones by the kidney, and it is often accompanied by an increase in blood pressure and other cardiovascular complications (24, 25). Calcium and phosphorus disorders are also common. One consequence of these anomalies is osteoporosis among adults (26). Kidney disease may affect erythropoietin (EPO) secretion, and thus the development of anemia may occur (27). Malnutrition is also widespread (4). Other signs, such as neurological disorders, may occur at very advanced stages of the disease (4).
Diabetic nephropathy is one of the most common complications of diabetes and is largely at the top of the leading causes of end-stage renal disease. Epidemiological evidence from many countries shows a significant increase in the number of dialysis patients with diabetes (28). Hyperglycemia, hypertension, smoking, and dyslipidemia are considered risk factors for CKD patients with diabetes (29). Chakkarwar et al. (30) showed that chronic smoking plays an important role in the progression of diabetic nephropathy. Tofovic et al. (31) showed that excessive consumption of caffeine worsens the state of kidney failure in obese and diabetic rats. Ritz et al. (32) showed that anemia is more common in diabetics than in non-diabetics, regardless of their glomerular filtration rate.
2. Objectives
The purpose of this study is to describe the prevalence, causes, and consequences of CKD and diabetic nephropathy on haemodialysis patients in Lebanon.
3. Patients and Methods
3.1. Participants
This cross-sectional survey was conducted between June 2015 and January 2016. A multi-stage stratified cluster sampling procedure was employed to select a representative sample of the primary care population in Lebanon. Two hospitals in the city of Nabatieh (south of Lebanon) and four in Beirut city were randomly chosen in proportion to the population size of each city. In this study, all the hemodialysis patients found in these hospitals (380 patients over the age of 18) are included, regardless of their socioeconomic status or their nationality. After obtaining permission to conduct the study in the hospital, we first obtained a list of the patients’ names and the calendar of dialysis sessions for each patient. Second, data was collected from patients during their dialysis sessions. To ensure good communication, the selected patients had to have good cognitive, physical, and psychic abilities. Patients who did not meet the inclusion criteria, or those who were suffering from acute diseases, were excluded from the study.
3.2. Measurements
A questionnaire composed of four main parts was developed using previous questionnaire-based research (33, 34). The first part focused on the demographic and general characteristics of participants, such as age, gender, education level, family history with CKD, and past body mass index. The second part included questions on behavioral history of patients and their quality of life (e.g., smoking and sleep quality) with a food frequency questionnaire (water, protein, salt, and caffeine). The third part aimed to determine the patients’ medical history in order to verify if they were affected by diabetes, diabetic nephropathy, hypertension, polycystic kidney disease, glomerulonephritis, lupus erythematosus, kidney stones, and repeated urinary infections. The last part focused on the consequences of the CKD on patients’ health, such as the development of anemia, cardiovascular disease, edema, osteoporosis, hypertension, and malnutrition. The questions were designed to be true/false or single-word answers to eliminate the need for patient explanations.
3.3. Statistical Analysis
Statistical analyses were conducted using SPSS software (version 20.0). The level of significance was set at P < 0.05 for all statistical analyses. Descriptive analyses were based on frequencies and percentages. The association between gender and each risk factor was assessed by bivariate analyses: the chi-square test was used to study the relationship with qualitative variables and t-student with continuous quantitative variables. The nutritional analysis of patients’ dietary history was analyzed by a dietitian (one of the authors: F. Karaki). The odd ratio measured the associations of CKD consequences with male and female gender from one side and various age groups from the other side.
4. Results
This study concerns patients at the last stage of chronic kidney disease who regularly undergo hemodialysis sessions.
4.1. Characteristics and Behavioral History Before CKD
Men made up 57.9% of the patients, and 42.1% were women. Among 380 patients, 340 patients (representing 89.5%) were over the age of 40, while the others were younger. The study of the level of education showed that 52.6% of the patients had a low level (e.g., illiterate or did not proceed beyond primary education), 42.1% had a medium level (secondary education), and 5.3% had a high level of education (university level). Although CKD can be transmitted hereditarily, our results showed that only 28.9% of patients had at least one family member who suffered from CKD. More than half of the patients had a BMI greater than 25 kg/m2 (21.1% and 34.2% were overweight or obese, respectively). As for smoking, 57.9% of patients were smokers of cigarettes or Shisha, or had been smokers. Among 380 patients, 220 patients (representing 57.9%) reported that they slept between six and eight hours a day, 90 subjects (representing 23.7%) slept more than eight hours, and 70 subjects (representing 18.4%) slept less than six hours. Concerning water consumption, 47.4%, 39.5%, and 13.2% of the patients reported that they drank less than two liters per day, more than three liters, or between two and three liters, respectively. Table 1 shows the repartition of these results according to gender.
| Male (n = 220) | Female (n = 160) | P Value | Total (n = 380) | |
|---|---|---|---|---|
| Age | 0.001 | |||
| < 40 | 20 (9.1) | 20 (12.5) | 40 (10.5) | |
| ≥ 40 | 200 (90.9) | 140 (87.5) | 340 (89.5) | |
| Education level | 0.191 | |||
| Low | 100 (45.5) | 100 (62.5) | 200 (52.6) | |
| Medium | 100 (45.5) | 60 (37.5) | 160 (42.1) | |
| High | 20 (9.1) | 0 | 20 (5.3) | |
| Family history of renal failure | 60 (27.3) | 50 (31.3) | 0.796 | 110 (28.9) |
| CKD duration in months (95% CI) | 21.3 - 53.8 | 11.1 - 25 | 0.049 | 19.4 - 39.4 |
| Height in cm (95% CI) | 161.7 - 176.2 | 155.6 - 164.4 | 0.053 | 160.5 - 169.8 |
| Weight in kg (95% CI) | 72 - 90.9 | 62.7 - 78.3 | 0.085 | 70.5 - 83.2 |
| BMI | 0.862 | |||
| Underweight | 20 (9.1) | 0 | 20 (5.3) | |
| Normal | 80 (36.4) | 70 (43.7) | 150 (39.5) | |
| Overweight | 40 (18.2) | 40 (25) | 80 (21.1) | |
| Obese | 80 (36.4) | 50 (31.3) | 130 (34.2) | |
| Smoking status | 150 (68.2) | 70 (43.7) | 0.139 | 220 (57.9) |
| Sleep duration (hours) | 0.363 | |||
| < 6 | 30 (13.6) | 40 (25) | 70 (18.4) | |
| 6 - 8 | 130 (59.1) | 90 (56.3) | 220 (57.9) | |
| > 8 | 60 (27.3) | 30 (18.7) | 90 (23.7) | |
| Water intake (liters per day) | 0.665 | |||
| < 2 | 110 (50) | 70 (43.8) | 180 (47.4) | |
| 2 - 3 | 30 (13.6) | 20 (12.5) | 50 (13.2) | |
| > 3 | 80 (36.4) | 70 (42.8) | 150 (39.5) |
aValues are expressed as No. (%).
4.2. Medical History Before CKD
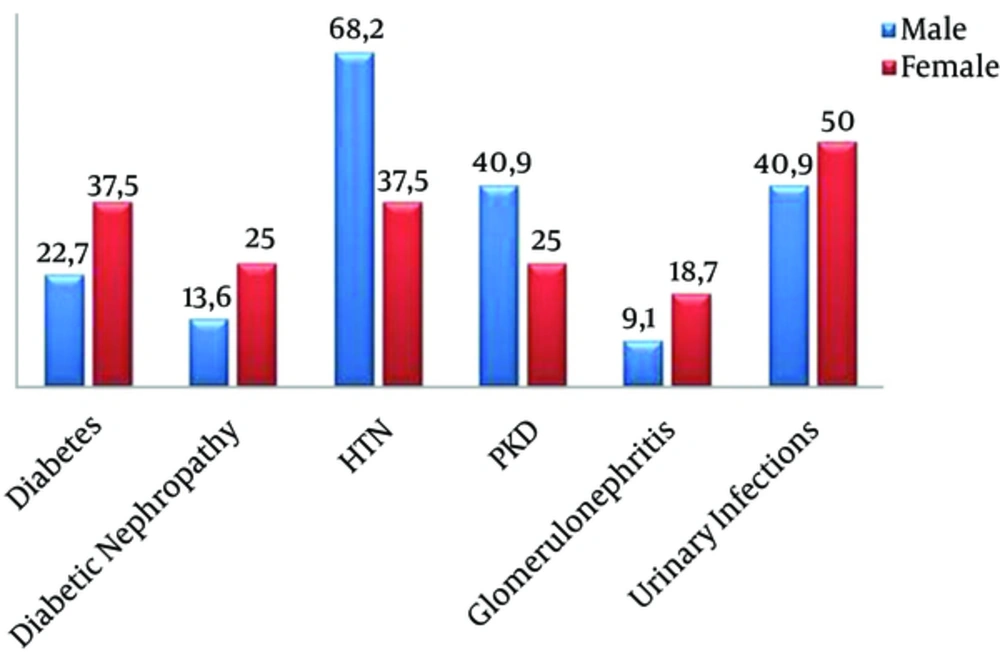
Chronic renal failure is a disease affected by genetic, behavioral, and pathological factors. The major pathological cause of CKD was hypertension (55.3% of patients), followed by polycystic kidney disease and repeated urinary tract infections (34.2% of patients), urinary tract obstruction by kidney stones (26.3%), diabetic nephropathy (18.4%), glomerulonephritis (13.2%), and lupus erythematosus (2.6%). Figure 1 shows the repartition of these results by gender.
4.3. Food History Before CKD
A balanced diet is essential to maintain kidney function. The study of the food history of the 380 patients with end-stage kidney disease showed that 52.6% of the patients consumed more than 56 g of protein per day, 31.6% of the patients exceeded the recommended daily intake of caffeine (400 mg/day), and 47.4% of patients consumed more than 2300 mg of sodium per day (Table 2).
| Recommended Dietary Allowance (RDA) per day | Percentage of Patients Below RDA, % | Percentage of Patients Above RDA, % | P Value | |
|---|---|---|---|---|
| Protein consumption per day | 0.8 g/kg = 56 g/d | 47.4 | 52.6 | 0.746 |
| Caffeine consumption per day | 400 mg/d | 68.4 | 31.6 | 0.023 |
| Sodium consumption per day | 2300 mg/d | 52.6 | 47.4 | 0.746 |
4.4. CKD Consequences
High blood pressure, cardiovascular disease, anemia, osteoporosis, malnutrition, and water retention (edema formation) are widespread consequences of chronic kidney disease. All of the 380 patients had permanent hypertension controlled by the regular hemodialysis sessions. A total of 55% of the patients suffered from anemia, 21% suffered from malnutrition, water retention, and edema formation, 18.4% had cardiovascular diseases, such as arteriosclerosis and myocardial damage, and 15.7% suffered from osteoporosis.
The repartition of results regarding gender represented in Table 3 shows that among hemodialysis patients, men are more prone to anemia, water retention, and osteoporosis than women (β = 0.231, P = 0.042; β = 0.347, P = 0.199; and β = 0.684, P = 0.671, respectively). However, women are more affected by cardiovascular disease and malnutrition (β = 2.059, P = 0.428 and β = 1.275, P = 0.767, respectively).
| Sex | Age Groups | |||
|---|---|---|---|---|
| Male (n = 220) | Female (n = 160) | ≥ 40 (n = 340) | < 40 (n = 40) | |
| Anemia | ||||
| No. (%) | 90 (40.1) | 120 (75) | 190 (55.9) | 20 (50) |
| Odd ratio (95% CI) | 1.000 | 0.231 (0.056, 0.950) | 1.000 | 1.267 (0.159, 10.074) |
| P value | 0.042 | 0.823 | ||
| Cardiovascular disease (myocardial disease or stroke) | ||||
| No. (%) | 50 (22.7) | 20 (12.5) | 40 (11.8) | 30 (75) |
| Odd ratio (95% CI) | 1.000 | 2.059 (0.345, 12.281) | 1.000 | 0.044 (0.004, 0.537) |
| P value | 0.428 | 0.014 | ||
| Edema (in lower limbs or lungs) | ||||
| No. (%) | 30 (13.6) | 50 (31.3) | 70 (20.6) | 10 (25) |
| Odd ratio (95% CI) | 1.000 | 0.347 (0.069, 1.742) | 1.000 | 0.778 (0.070, 8.669) |
| P value | 0.199 | 0.838 | ||
| Osteoporosis | ||||
| No. (%) | 30 (13.6) | 30 (18.8) | 30 (8.8) | 30 (75) |
| Odd ratio (95% CI) | 1.000 | 0.684 (0.119, 3.933) | 1.000 | 0.032 (0.003, 0.415) |
| P value | 0.671 | 0.008 | ||
| Malnutrition | ||||
| No. (%) | 50 (22.7) | 30 (18.8) | 50 (14.7) | 30 (75) |
| Odd ratio (95% CI) | 1.000 | 1.275 (0.256, 6.333) | 1.000 | 0.057 (0.005, 0.669) |
| P value | 0.767 | 0.023 | ||
Concerning age, anemia is more prevalent in younger patients (e.g., under 40 years) than in older ones (β = 1.267, P = 0.823), whereas cardiovascular diseases, water retention, osteoporosis, and malnutrition are most prevalent in people over the age of 40 years (β = 0.044, P = 0.014; β = 0.778, P = 0.838; β = 0.032, P = 0.008; β = 0.057, P = 0.023, respectively).
4.5. Diabetic Nephropathy
Among the 380 patients, 110 subjects had diabetes (representing 28.9%), of which 70 had diabetic nephropathy (suffering from diabetic retinopathy, representing 18.4% of hemodialysis patients). Diabetic nephropathy is one of the most common complications of diabetes and the top cause of end-stage renal disease. The results of this study show that patients with diabetic nephropathy adopted a diet rich in protein (71.4%) but less rich in caffeine (42.9%) and sodium (28.6%) than other patients’ diets. On the other hand, anemia (57.1%) and cardiovascular diseases (28.6%), such as arteriosclerosis and cardiac muscle atrophy, are more prevalent among patients with diabetic nephropathy than other patients. In contrast, the prevalence of water retention (14.3%), osteoporosis (14.3%), and malnutrition (0%) is lower among them (Table 4).
| CKD Consequences | CKD Cases not Caused by Diabetes, % | Diabetic Nephropathy Cases, % | ||
|---|---|---|---|---|
| Anemia | 54.8 | 57.1 | ||
| Cardiovascular disease | 16.1 | 28.6 | ||
| Edema | 22.6 | 14.3 | ||
| Osteoporosis | 16.1 | 14.3 | ||
| Malnutrition | 25.8 | 0 | ||
| % Below RDA | % Above RDA | % Below RDA | % Above RDA | |
| Dietary analysis | ||||
| Protein consumption per day | 51.6 | 48.4 | 28.6 | 71.4 |
| Caffeine consumption per day | 71 | 29 | 57.1 | 42.9 |
| Sodium consumption per day | 48.4 | 51.6 | 71.4 | 28.6 |
5. Discussion
A silent and alarming epidemic is growing that will affect millions of people around the world. Like high blood pressure or diabetes, end-stage renal disease develops without warning signs. Several studies on the epidemiology and the prevalence of CKD and diabetic nephropathy were made in different countries (11, 13, 28, 29, 32, 35). However, data about the Middle East are rarely available. In this article, we studied for the first time the prevalence, causes, and consequences of CKD and DN in hemodialysis patients in Lebanon.
In accordance with several studies already carried out (1, 2, 10, 18, 36), our study shows that advanced age, low education levels, a BMI over 25 kg/cm2, and smoking increase the prevalence of CKD. Contrary to what has been shown by Xue et al. (14), we noticed that men are more affected by CKD than women. This may be related to the protective effect of female hormones against renal aging (2). A study published in 2005 showed that CKD can be transmitted hereditarily, and a genetic abnormality can cause hyperuricemia, which can gradually damage renal function (37). However, in this study, more than a quarter of the patients had at least one family member with CKD. Salifu et al. showed that sleep disorders are associated with a high prevalence of CKD (17). However, our results do not validate this hypothesis, since 60% of hemodialysis patients reported that they slept between six and eight hours per day. The influence of the consumption of water was also studied. Our results were consistent with previous studies that show that low water consumption is a risk factor for CKD (19, 20).
In addition, other studies show that the most prevalent CKD risk factor is hypertension in South Africa and Congo (18, 38), glomerulonephritis in Bangladesh (11), and hyperlipidemia in China (14). In the current study, all of the 380 patients had permanent hypertension controlled by the regular hemodialysis sessions. In total, 55% of the patients suffered from anemia, 21% suffered from malnutrition, water retention, and edema formation, 18.4% had cardiovascular diseases, such as arteriosclerosis and myocardial damage, and 15.7% suffered from osteoporosis.
Regarding the relationship between sex and pathologies causing CKD, it was found that the prevalence of DN, glomerulonephritis, and urinary infections is higher in women than in men, yet hypertension and polycystic kidney disease are more prevalent in men. This is consistent with what has been shown in other studies (16, 39-41).
The analysis of the dietary history of hemodialysis patients shows no relationship between excessive intake of sodium and protein and the occurrence of CKD. The results show no decisive effect on the impact of proteins on glomerular function, as Fatehi-Hassanabad and Chan showed (21). These results contradict earlier studies indicating a positive or a negative effect of sodium on renal function (22, 23). The effect of caffeine on renal function in humans has not been studied. However, this study was able to show a significant relationship between caffeine intake and the occurrence of CKD.
The consequences of CKD depend on the gender and the age of patients. First, all hemodialysis patients suffer from hypertension caused by the accumulation of water in the body and the inability of the kidneys to excrete urine. Although anemia is usually more common in women, because of blood loss during menstruation, the results showed that men undergoing hemodialysis were more prone to anemia. This is probably due to the fact that most women in the study were menopausal as well as the men experiencing a loss of blood or iron deficiency due to a very restrictive diet (42). Also, water retention and the formation of edema are more common in men than in women. This is possibly related to the tendency of men to consume more salt and the fact that they are more susceptible to dehydration. In addition, men are more affected by osteoporosis related to CKD, probably because of their advanced age. Yet, women are more susceptible to cardiovascular disease, probably due to their sedentary lifestyle. Thus, malnutrition is more common in women, possibly because of the difference in the amount of lean mass in women. It is not surprising that the complications of CKD increase with age, especially cardiovascular disease, osteoporosis, and malnutrition. In contrast, the prevalence of anemia is higher at a younger age (< 40 years) due to the activity of female hormones and the monthly blood loss in women.
The study of diabetic nephropathy showed that almost 30% of patients were diabetic, but only 18% had diabetic nephropathy. It has been found that patients with DN have a higher risk for anemia and cardiovascular disease than others, because of the additional effect of diabetes, but a lower risk of having edema of the osteoporosis and malnutrition, for poorly understood reasons. The dietary history of DN patients showed that their diet was richer in protein and caffeine than other patients. Therefore, this diet can contribute to the acceleration of the progression of DN, then the damage to the glomerular filtration rate caused by diabetes. This is not the case for sodium, since these patients consumed a smaller amount than others.
This study was carried out to determine the prevalence, causes, and consequences of chronic kidney disease and diabetic nephropathy in Lebanon. Older age, low education levels, overweight and obesity, smoking, and consumption of an inappropriate amount of water are all factors that affect renal function and accelerate the progression of chronic kidney disease. In Lebanon, high blood pressure is the leading cause of end-stage kidney disease, followed by polycystic kidney disease and repeated urinary infections, urinary tract obstruction by kidney stones, diabetic nephropathy, glomerulonephritis, and finally lupus erythematosus. High blood pressure, anemia, malnutrition, water retention, cardiovascular disease, and osteoporosis are common consequences that aggravate the state of chronic kidney disease patients. This high prevalence of behavioral and pathological factors, and the resulting consequences of CKD, suggest a major awareness of the Lebanese population of the risks of chronic kidney disease and the necessity of the prevention of CKD by following a healthy lifestyle and managing any existing diseases.