1. Background
Post-operative pain (POP) is endured by millions every day all over the world and its effective relief is very important and necessary (1, 2). POP imposes additional costs on the health care system every year and it is an important aspect considered in the healthcare process (3, 4). Inappropriate pain management in different settings, from the emergency department to the operating room and afterward, has been documented in the current literature and research regarding better approach is still ongoing (5-8). The efficient management of POP is currently a part of surgery process and reduces patients’ morbidity as well as their mortality, accelerates recovery and discharge from the hospital, improves patients’ quality of life, and reduces costs. The efficient management of POP includes a multimodal approach during which various drugs with different mechanisms and administration routes are used. One of the aspects considered in this regard is the social history of the patient. A history of drug abuse is one of the most important things that should be recorded in patients’ medical profile since opium addict patients make up a large portion of hospital visitors and obviously, their pain threshold and need for analgesia are different (9-11). Addiction is an international problem and one of the healthcare problems in various societies and there may be only a few countries not having this issue. Addiction is one of the 4 major crises that has caused serious harm to at least 200 million people around the world (9, 10, 12). The prevalence of drug abuse such as opioid use is on the rise, and a considerable percentage of opioid-addict patients are admitted for surgery. Some authors believe that these patients can be expected to the experience of increased postoperative pain, greater postoperative opioid consumption, and prolonged use of healthcare resources for managing their pain (13). Dexmedetomidine (Dex) is one of the drugs considered for POP in opium addict patients in recent studies. This drug is a highly selective α2-adrenergic and has been used as an analgesic along with other anesthetic drugs for reducing POP in opium-addict patients (14). Achieving adequate pain control in opioid addict patients can be challenging because commonly used strategies for alleviating postoperative pain may have diminished effectiveness (13). There are limited available scientific sources of data and rare controlled studies to guide the anesthesiologist regarding preoperative analgesic care in opioid addict patients, despite the increasing prevalence of opioid dependence (15).
2. Objectives
In the present study, we aimed to evaluate the effect of using Dex during femoral neck surgery on postoperative analgesic consumption in opium addict patients.
3. Patients and Methods
The present study is a double-blind randomized clinical trial carried out in Imam Hossein teaching Hospital, Tehran, Iran, in 2015.
3.1. Study Population
In this study, patients aged between 18 to 75 years with a proximal fracture of the femur and a history of inhaled or oral opium abuse for 6 consecutive weeks who were candidates of femoral neck surgery using spinal anesthesia were included. Pregnant and lactating women, patients with a history of chronic pain and use of analgesics, and those with a body mass index (BMI) more than 35 were excluded from the study. History of diabetes, myocardial infarction, cerebrovascular accidents, hypertension, malignancy, renal failure, hepatic dysfunction, coagulation disorders, history of allergy to drugs, and the need for blood transfusion during surgery were also considered as the exclusion criteria. We should note that patients who could not bear the spinal anesthesia for any reason and needed general anesthesia were also excluded from the study.
3.2. Randomization and Blinding
Demographic data and baseline characteristics of the patients were gathered using a pre-designed checklist. In this study, patients were randomly allocated to 2 groups of intervention and control. Using the random numbers table, each patient was given a number and accordingly was allocated to one of the groups. Patients were blinded to the administered drug and the anesthesiologist and the surgeon were blinded to the grouping of patients. Another physician who did not have any role in the study process prepared the intravenous (IV) drug infusion pump. In the intervention group, Dex solution in normal saline was infused while in the control group, normal saline alone was infused for blinding and preventing information bias.
3.3. Intervention
To induce spinal anesthesia, 20 mg hyperbaric bupivacaine was injected into the intervertebral space L3 - L4 or L4 - L5 of patients using 25-gage needle and after block stabilization, IV midazolam with 0.02 mg/kg dose was administered. Each one milliliter of Dex (100 µg) was diluted to 1 µg/cc concentration and infused with 0.5 µg/kg/h dose in the intervention group. In control group, normal saline was administered to the patients with a 0.5 cc/kg/h infusion rate. After the surgery, the pain intensity of the patients in recovery was assessed in 10-minute intervals based on the visual analog scale (VAS) until 2 hours postoperatively. If VAS > 3, morphine sulfate was injected with 2 mg dose and repeated until reaching a maximum of 15 mg if needed. In the orthopedic department, the patients underwent infusion of pain pump including 2 and locked-out interval injections for about 15 min by the patients and the used content was calculated in 2 - 6, 6 - 12, 12 - 18, and 18 - 24 hours postoperatively to determine the dose of opium used.
3.4. Sample Size
Since no reliable similar study was done up to the time of proposal preparation, to reduce sampling error, first a sample of 10 patients was drawn with the same inclusion and exclusion criteria and participants were randomly allocated to the 2 mentioned groups. Then, considering the mean difference of need for analgesics between the 2 groups and considering α = 0.05 and β = 0.2, a sample size of 16 patients in each group was calculated. Considering the probability of loss or the exclusion of some patients, 30 subjects were included in each group.
3.5. Statistical Analysis
Data gathered from the patients were entered into SPSS version 22. Mean and standard deviation (SD) were used to describe quantitative data and qualitative data were described using frequency and percentage. To compare means of quantitative data in the 2 groups, Unpaired t-test was used and if data distribution was not normal, the non-parametric Mann-Whitney-U test was applied. To compare qualitative data in the 2 groups, Pearson Chi-Square and if needed Fisher-Exact-Test were used. All statistical tests were carried out as 2-tailed at the significance level of 0.05. Confidence interval (CI) of 95%, type 1 error of 0.05, and type 2 error of 0.2 were considered.
3.6. Ethical Issues
This study was done after receiving approval from the ethics committee of Shahid Beheshti University of Medical Sciences under the number IR.SBMU.MSP.REC.1395.22. All the patients participated after they received a thorough explanation regarding the process of the study and giving a written consent. Adhering to the principles of Helsinki declaration and keeping patient data confidential were among the other measures taken for maintaining ethical conduct in this study. The protocol of the study was registered on the Iranian registry of clinical trials (www.IRCT.ir) under the code number IRCT201608019593N4.
4. Results
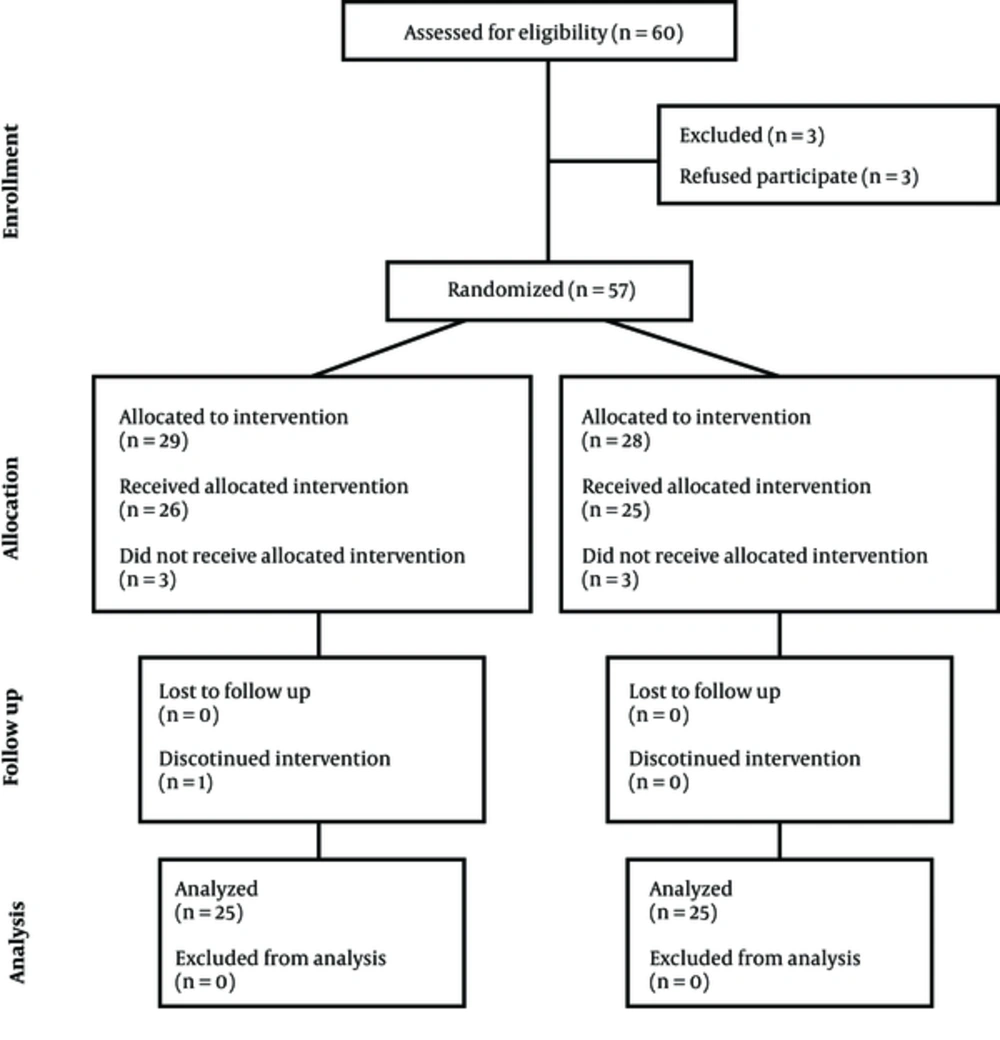
Totally 60 patients met the inclusion criteria of the study, 3 of which did not give consent for participation and therefore, 57 patients were enrolled. Thereafter, 2 out of the 29 patients in the control group were excluded due to unsuccessful spinal block; in addition, due to the long interval between the block and surgery initiation, another patient was excluded, too. Moreover, 1 patient was excluded due to excessive bleeding and need for blood transfusion. On the other hand, 3 of the 28 patients allocated to Dex group were also excluded. 2 of them had an unsuccessful spinal block and 1 was very nervous since the beginning of the operation and therefore, received propofol infusion for inducing sedation. Consequently, the study was done with 25 patients in each group (Figure 1).
4.1. Baseline Findings
Table 1 depicts demographic and baseline data of the study patients including mean age, mean BMI, duration of addiction, mean daily dose of opium used, duration of operation, systolic blood pressure (SBP) before spinal analgesia, and SBP before drug infusion in the 2 groups, which were not significantly different (P > 0.05).
| Variable | Group | P Value | |
|---|---|---|---|
| Dexmedetomidine | Control | ||
| Sex (male: female) | 22: 3 | 23: 2 | 0.63 |
| Age (year) | 56.92 ± 16.80 | 58.08 ± 13.95 | 0.90 |
| Body mass index (kg/m2) | 25.27 ± 3.17 | 23.99 ± 2.69 | 0.13 |
| Addiction duration (year) | 17.08 ± 12.56 | 16.60 ± 10.04 | 0.91 |
| Mean daily opium consumption (gram) | 1.88 ± 0.75 | 1.92 ± 0.84 | 0.96 |
| SBP before spinal anesthesia (mmHg) | 122.76 ± 9.45 | 124.32 ± 10.83 | 0.59 |
| SBP before drug administration (mmHg) | 112.76 ± 7.49 | 114.88 ± 12.51 | 0.49 |
| Duration of surgery (minute) | 130.80 ± 14.97 | 129.60 ± 15.13 | 0.69 |
Abbreviation: SBP: systolic blood pressure.
4.2. Time to Pain
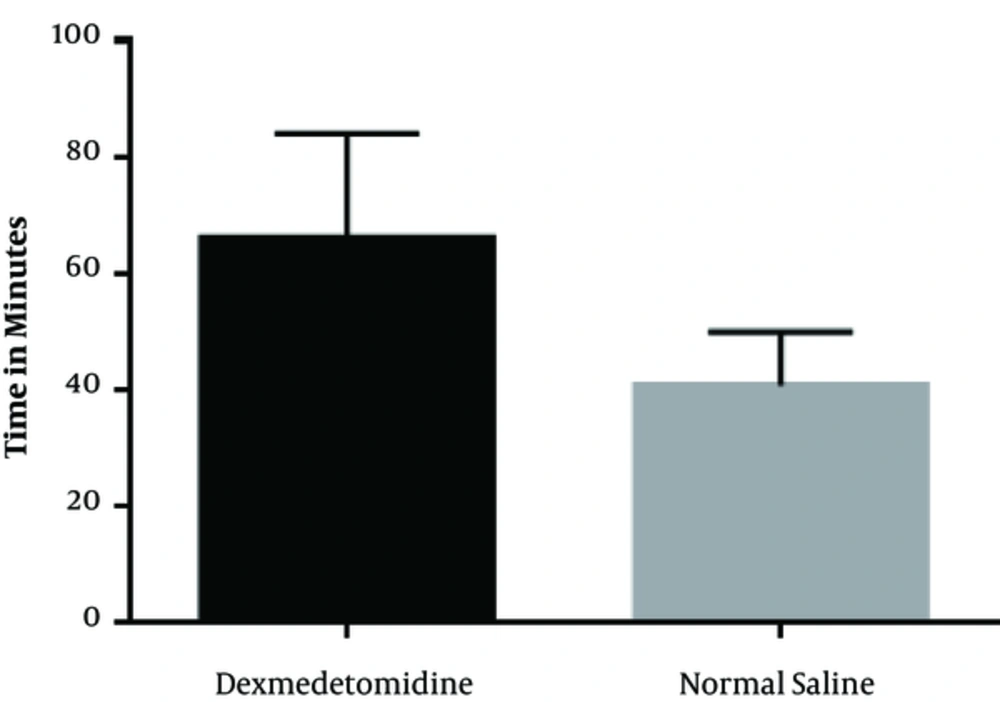
In the Dex group, the mean time interval between the end of the surgery and patients’ request for receiving opioids in recovery was 66 ± 18.4 minutes, while it was 40.6 ± 9.36 minutes in the normal saline group, which showed a significant difference as presented in Figure 2 (P < 0.001).
4.3. Opioid Consumption
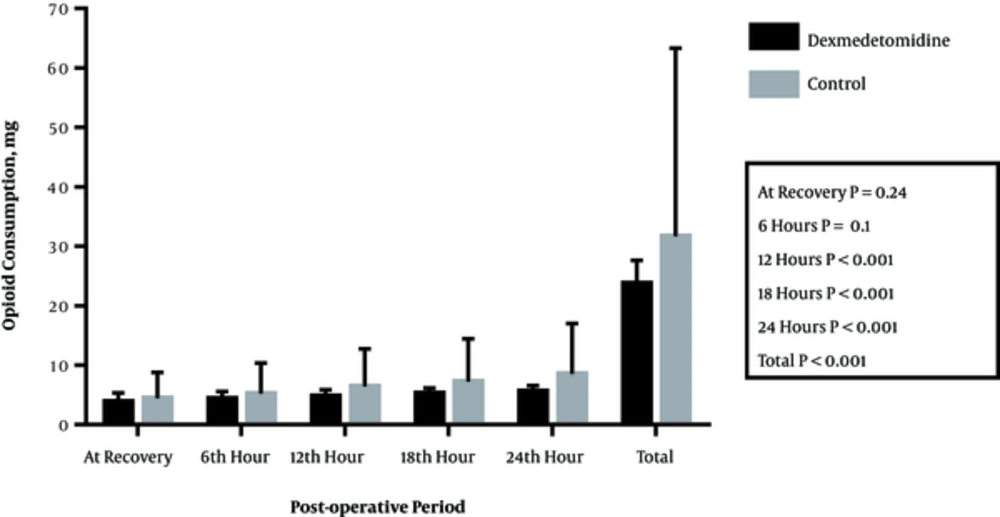
Figure 3 and Table 2 show the amount of morphine needed in recovery until 2 hours postoperatively along with 2 - 6, 6 - 12, 12 - 18, and 18 - 24 hour intervals postoperatively in the 2 groups. The difference between the 2 groups in this regard was not significant until 2 hours of recovery postoperatively (P = 0.24); however, in the other time intervals, the differences were significant. The total use of morphine during 24 hours postoperatively was 23.7 ± 3.97 mg in the Dex group and 31.67 ± 2.41 mg in the normal saline group, which showed a significant difference (P < 0.001).
| Time | Group | P Value | |
|---|---|---|---|
| Dexmedetomidine | Control | ||
| At recovery | 3.84 ± 1.52 | 4.4 ± 1.63 | 0.24 |
| 2 - 6 hours | 4.33 ± 1.27 | 5.19 ± 1.01 | 0.01 |
| 6 - 12 hours | 4.75 ± 1.12 | 6.37 ± 0.70 | < 0.001 |
| 12 - 18 hours | 5.22 ± 0.96 | 7.22 ± 0.80 | < 0.001 |
| 18 - 24 hours | 5.56 ± 1.01 | 8.49 ± 0.36 | < 0.001 |
| Total | 23.70 ± 3.91 | 31.67 ± 2.41 | < 0.001 |
5. Discussion
Based on the results of the present study, the mean time interval between the end of the surgery and request for opioids was significantly higher in the Dex group than in the normal saline group. Although the mean morphine use in recovery until 2 hours postoperatively was higher in the normal saline group, the difference was not statistically significant. However, the mean morphine use was significantly lower in the Dex group at the other time intervals. Additionally, the mean total morphine use during 24 hours postoperatively was also significantly lower in the DEX group compared to the control group.
The effect of premedication with α2 agonist on postoperative complications in addict patients has been poorly studied. The results of a study by Jabbary Moghaddam et al. showed that clonidine as premedication can be an effective drug to decrease the incidence of shivering and recovery time after anesthesia for leg fracture operations not only in patients without addiction but also in opium addicted ones whose postoperative complications make a great challenge for anesthesiologists (16).
In one study, Kaya et al. evaluated the effect of Dex infusion during surgery on the need for morphine sulfate in patients undergoing major abdominal surgeries. In that study, the morphine use was lower in the Dex group 2 and 48 hours postoperatively. In addition, the trend of changes in the morphine use by patients to reduce pain had a steeper slope in the placebo group and showed a slower increase in the Dex group, which is in line with the present study (17).
In 2004, a study was done on the effect of Dex and morphine on analgesia after major surgeries. 34 patients in 2 groups were evaluated regarding hemodynamic changes during operation, sedation and analgesia rates during surgery, and additional morphine use postoperatively. The main finding of the study was that in the Dex group patients, clearly less morphine was used during recovery (18).
In another study, Annamalai et al. evaluated the effect of IV Dex on spinal anesthesia with hyperbaric bupivacaine in 2014. The mean total use of analgesics during 24 hours postoperatively was lower in the Dex group than in the normal saline group and the difference was statistically significant. Analgesics in the study were paracetamol, diclofenac, and tramadol (19).
A number of studies have been carried out on Dex in recent years proving the effect of this drug on extending sensory blockade and even some motor blockades. However, there has not been any study in opium addict patients regarding the effects of Dex. As shown in the present study, the amount of morphine sulfate used postoperatively reduced in the addicts receiving IV Dex during surgery. This means that our results are similar to the results of other studies regarding the effect of Dex on the quality of spinal block in terms of sensory block duration. Although not tested in our study, a less need for morphine postoperatively in the Dex group could prove the prolonged duration of sensory blockade (20).
Unlugenc et al. conducted a prospective, randomized, double-blind, controlled study to examine the effect of pre-anesthetic administration of Dex. They reported that a single IV dose of Dex given 10 minutes before induction of anesthesia significantly reduced postoperative morphine consumption at identical pain scores (21).
The efficacy of IV Dex in prolonging the analgesic duration of brachial plexus block for shoulder surgery was assessed in a randomized clinical trial and it was found that Dex reduced the pain and opioid consumption up to 8 hours postoperatively and did not prolong the duration of the motor blockade (22).
Lin et al. claimed that the addition of Dex to IV morphine contained patient-controlled analgesia resulted in superior analgesia, less morphine consumption, and lower morphine-induced side effects (23).
There are still considerable disagreements on the effect of Dex Infusion during surgery on hemodynamic parameters (24, 25). Therefore, it is suggested to carry out further studies and consider non-addicted participants in need of surgery as well as addicted ones to study the effect of Dex on hemodynamic parameters changes and compare such variables between them. Taking more variables such as cardiac output and ejection fraction into consideration would raise the value of such studies.
5.1. Limitation
One of the limitations of this study was that the study population consisted of opium addicts and although they were given a thorough explanation of the study process and written consent was obtained from them, we cannot be 100% sure that they have not used opium postoperatively. On the other hand, there is no standard and reliable method to properly select these patients for participation in studies regarding the definition of addiction considering the parameters of drug abuse rate, duration of addiction, and potential effects of drugs on them that can affect the study results. As mentioned before, the definition of addiction in DSM-5 does not include these items but mostly evaluates qualitatively emotional, mental, and behavioral problems in the addicts. It is suggested to carry out more studies, considering non-addict patients in need of surgery along with addicts regarding the effects of DEX on hemodynamic changes and pain and compare the results.
5.2. Conclusion
It is likely that IV Dex infusion during femoral neck fracture surgery under spinal anesthesia leads to less opioid use within 24 hours postoperatively in opium addict patients.