1. Background
A psychoactive amphetamine derivative used worldwide as a recreation is 3, 4-Methylenedioxymethamphetamine (MDMA or “ecstasy) (1). The Greek word “εκστασις” (ekstasis) means “standing out of yourself”, and the name ecstasy is used to mention the main effects of the drug. Euphoric feelings and the ability to socialize usually increase after taking of MDMA. In addition, one may experience entactogenic effects and feel highly connected with others (2). The abuse of MDMA is a significant public health problem. Despite this popular misconception that ecstasy is “safe”, it causes cardiac arrhythmias, hypertension, hyperthermia, serotonin syndrome, hyponatremia, liver injury, Testicular atrophy, seizures, coma, and in some cases, death (3). In 2012, global consumers of MDMA aged 13 - 34 years were estimated to range between 9.4 and 28.2 millions (4). In the reproductive system, complaints about erectile dysfunction (5) or lack of sexual drive (6) are frequent among the various subjective symptoms reported by the drug consumers. MDMA interrupts the hypothalamic-pituitary-gonadal reproductive axis (7) and also causes endocrine alterations, increasing corticotropin (ACTH), cortisol, and prolactin (8). It is suggested that there is a functional relationship between the increase of prolactin and lack of sexual drive after acute exposure to MDMA (9). MDMA increases reactive oxygen species (ROS) (10); therefore, unspecific mechanisms such as oxidative stress or genotoxicity exert direct effects on reproductive organs (11). In addition, in young male rats, DNA damage in sperm is increased, and testes histopathology are changed after chronic exposure to MDMA (12).
Vitamin E is a lipid-soluble vitamin that was discovered by Evans in 1922 (13). It protects membrane stability against free radicals-induced peroxidation (14). In the reproductive system, the adverse effects of reactive oxygen species can be compensated by Vitamin E (15).
So far, to the authors’ knowledge, there has been no information about the effect of vitamin E on the reproductive system toxicities induced by MDMA exposure in mice or other mammals. Therefore, this study was conducted to analyze protective effects of Vitamin E against damages induced by MDMA exposure in sperm parameters. In addition, the antioxidant status was analyzed. To simulate the human MDMA exposure, the administration schedule was a single daily dose, three sequential days/week for 5 weeks (16).
2. Objectives
The current study was an attempt to assess the protective effects of Vitamin E on serum biochemical indices and sperm quality parameters in MDMA treated mice.
3. Materials and Methods
3.1. Animals and Treatments
The internationally accepted principles for laboratory animal use and care as found in the US guidelines (NIH publication# 85 - 23, 1985) were applied to handle all the procedures. Moreover, the protocol was approved by the committee on ethics in animal experimentation of Urmia University of Medical Sciences. Twenty eight sexually matured, 6 - 8-week-old male albino mice with weight range of 25 to 30 g were purchased from the animal house of Urmia University of Medical Sciences. Standard animal house conditions of illumination (12 h light: 12 h dark photoperiod), ventilation (kept in a room with a temperature of 25°C), and free access to water and food were taken into account in maintaining four mice per cage. The mice were randomly assigned to 4 treatment groups (7 mice in each). Group 1 (Control) received saline (0.9% NaCl) by gastric gavage and i.p.; Group 2 (MDMA) received pure MDMA (10 mg/kg) (BiotechnologyUnit of Iran Medical Sciences University, Tehran) dissolved in saline (0.9% NaCl) (7) i.p. and saline (0.9% NaCl) by gastric gavage; Group 3 (MDMA + Vitamin E) received pure MDMA (10 mg/kg) dissolved in saline (0.9% NaCl) i.p.,and vitamin E (150 mg/kg) (Sigma, USA) having been dissolved in olive oil (17) was gavaged; and Group 4 (olive oil) received olive oil (150 mg/kg) by gastric gavage and saline (0.9% NaCl) i.p. MDMA and dose selection of vitamin E was based on previous reports,respectively (16, 18), in accordance with duration of spermatogenesis in mice which is 35 days. At the end of the five weeks exposure and consequently after 24 hours of the last administration, a mixture of ketamine and xylazine (100/10 mg/kg, i.p.) was used to anesthetize the mice. Through cardiac puncture in heparinised tubes, blood samples were collected. Then, the samples were centrifuged for 5 minuts at 4 - 6°C and 2,500 rpm, and the obtained plasma was stored at -80°C until it was used for biochemical indices assays. After collecting blood samples, the animals were sacrificed by cervical dislocation. Cauda epididymis of the animals was dissected and used for sperm parameters analysis.
3.2. Biochemical Indices
3.2.1. Determination of Malondialdehyde (MDA)
The lipid peroxidation level in sperm homogenate was measured as malondialdehyde (MDA), which is the end product of lipid peroxidation and reacts with thiobarbituric acid (TBA) to produce TBA reactive substance (19).
Malondialdehyde was measured in plasma by a commercial kit (Bioassay Technology Laboratory, China) based on the kit guidelines.
3.2.2. Determination of Total Antioxidant Capacity (TAC)
The total antioxidant capacity (TAC) of plasma from all groups was measured. This test was based on the assessment of ferric reduction antioxidant power (FRAP) (20).
TAC of plasma was determined by an LDN (Labor Diagnostitika Nord) commercial kit according to the kit guidelines.
3.3. Sperm Parameters Analysis
3.3.1. Sperm Sampling
A 60 mm petri dish containing medium 1 mL Human Tubal Fluid (HTF; Sigma, St. Louis, USA) and 4 mg mL-1 bovine serum albumin (BSA; Sigma, St. Louis, USA) was used to examine sperm parameters of the sperm sampled from Cauda epididymis of each animal. Both epididymis (caudal region) of each animal were transferred to medium pre-warmed to 37°C. The caudate was minced making 5 - 7 slashes with a 30-gauge needle of an insulin syringe in order to swim out the sperm into the medium. After incubation for 30 minutes at 37°C in 5% CO2, spermatozoa were released from epididymis (21).
3.3.2. Sperm Count
A 1:20 dilution was prepared for counting sperms; 10 of diluted sperm from 10 μL sperms added to 190 μL distilled water was dropped on a Neubauer slide on which a stone cover slip had been placed before and finally the average number of sperms was counted (22).
3.3.3. Sperm Motility
To evaluate sperm motility, 10 μL sperm suspension was placed on the Neubauer slide and the percentage of sperm motility was evaluated under a light microscope with a magnification of 20X (23).
3.3.4. Sperm Viability
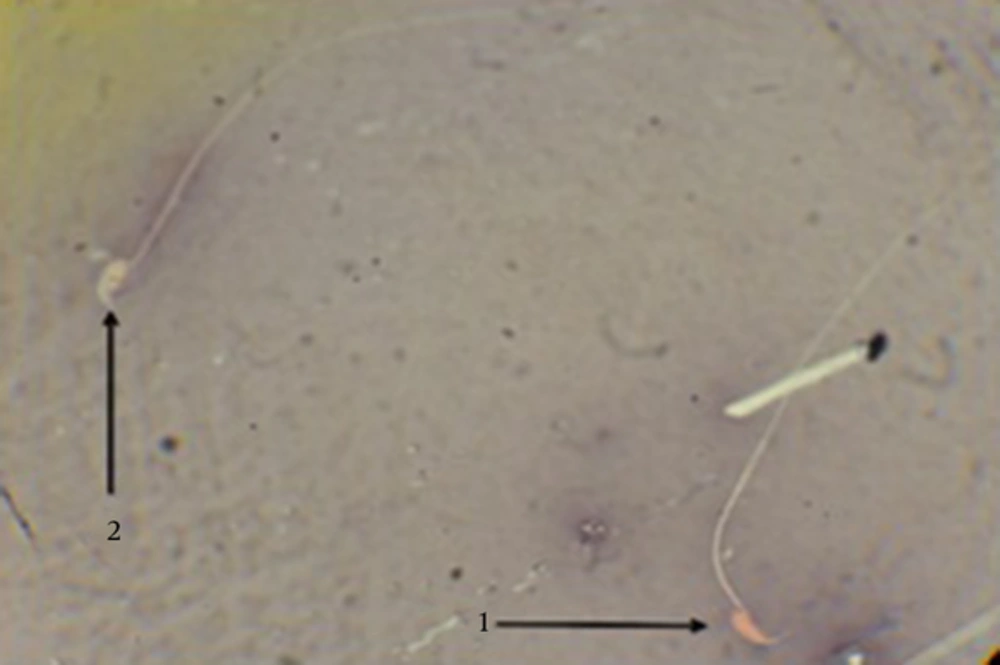
Twenty microliters of the sperm sample was placed on a clean slide and then 20 μL eosin solution was added. 20 μL nigrosin was further added after 20 - 30 seconds. Then, the smears and drying slides were prepared and finally alive (colorless) and dead (colored with eosin) spermatozoa percentages were determined by a light microscope (at magnification of 10-40X) (21).
3.3.5. Sperm Morphology
In order to evaluate sperm morphology, aniline blue (AB) staining method was applied and abnormal morphologies percentage, particularly the cytoplasmic residual of sperms that was considered as abnormal morphology, was determined (24).
3.4. Evaluation of the Level of DNA Strand Damage
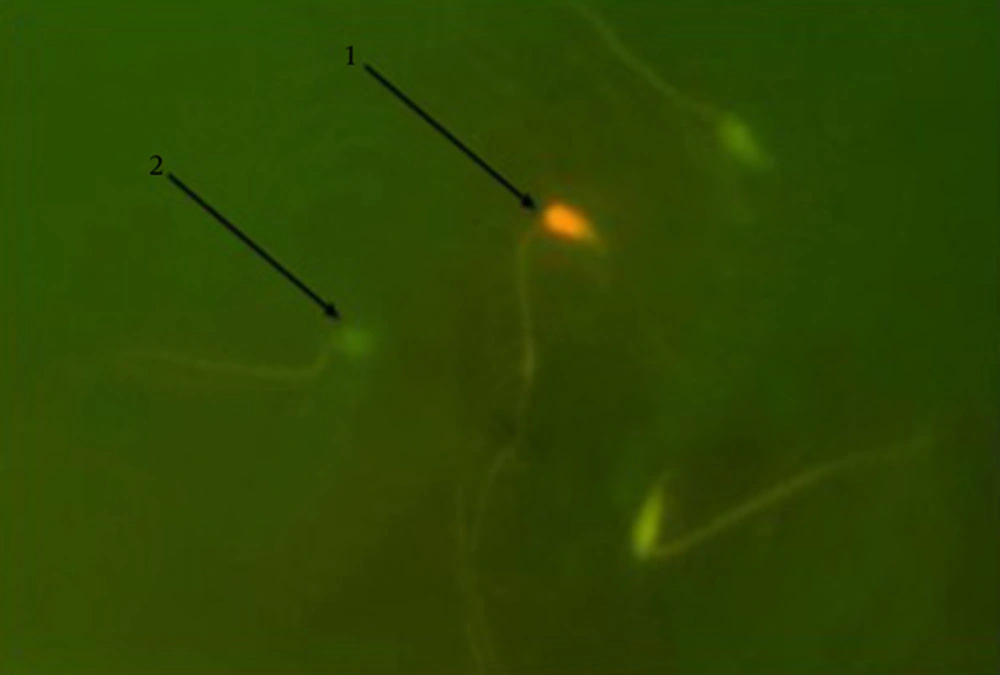
Acridine orange staining was used to estimate any kind of breakage in mice sperms DNA double strands. Based on the level of damage, DNA damage was observed as yellow to red fluorescence while the normal sperms were observed as green fluorescence (25). Thick smears as usual in this method for fixation were placed in carnoy’s fixative (methanol/acetic acid 1:3) for 2 hours. The slides were then left, for 5 minutes, at laboratory temperature to be dried. The slides were sited in a dark place including 1 mg of AO in 1000 mL distilled water. The stained solution was produced then itcontained 10 mL of the stock which was added to 40 mL 0.3 M NaHPO4.7H2O solution (26). After 5 min of staining, the slides were assessed using a fluorescence microscope with a 100 × lens and finally, the results were reported as percentage.
3.5. Sperm Nucleus Maturity Evaluation
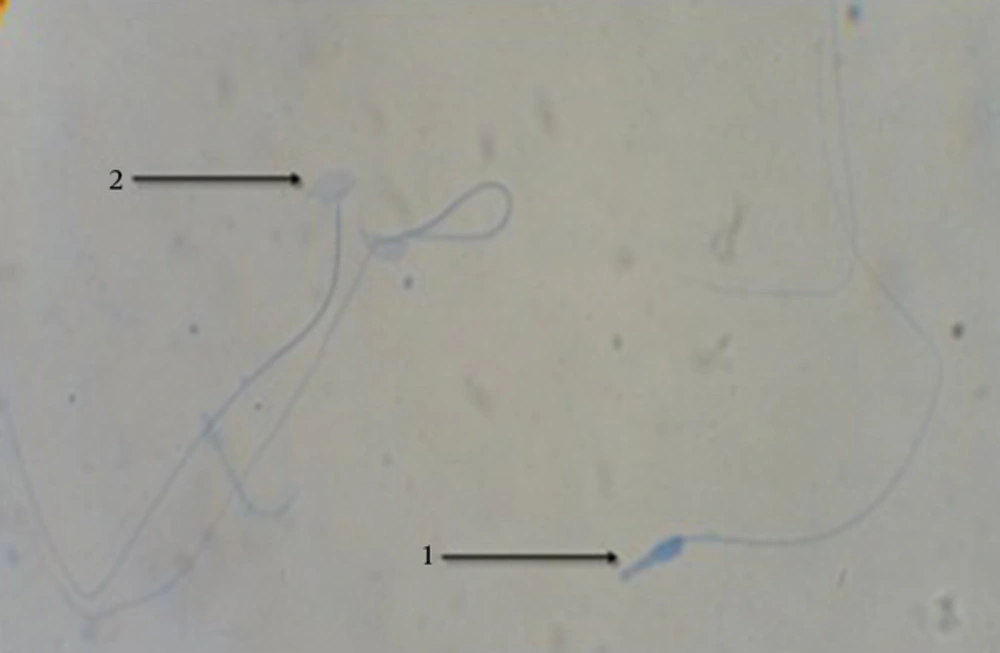
Aniline blue staining method was applied to evaluate the sperm nucleus maturity. In this staining method, immature sperms with high histone showed grayish dark blue in their nucleus and mature sperms were pale. In 30 minutes, sperm samples fixation was performed in glutaraldehyde 3% in saline phosphate buffer (pH = 7.2). Then, the slides were placed for 7 minutes in aniline blue solution and were examined by alight microscope with 10 × 100 magnification after drying. Final results were reported as percentage (27).
3.6. Statistical Analysis
SPSS version 16 was used for all Statistical analysis (P < 0.05).Summary statistics were presented as Mean ± SD In addition, one way ANOVA test as well as fullow up Tukey test were used to find the effects andpair–wise comparisons, respectively.
4. Results
4.1. Biochemical Indices
4.1.1. Total Antioxidant Capacity (TAC)
Biochemical analyses indicated a significant decrease (P < 0.05) in the serum TAC in the MDMA- group compared to the other groups. However, the other groups did not have any significant differences with each other (Table 1).
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | MDMA | MDMA + Vitamin E | Olive Oil | |
| TAC, mmol/L | 0.53 ± 0.06b | 1.25 ± 0.17a | 1.01 ± 0.15a | 1.27 ± 0.12a |
| MDA, µmol/L | 1.65 ± 0.13a | 2.25 ± 0.14b | 1.82 ± 0.18a | 1.63 ± 0.20a |
Abbreviations: MDA, malondialdehyde; TAC, total antioxidant capacity.
aValues are expressed as mean ± SD.
bDifferent letters in each row represent significant differences between the groups (P < 0.05).
4.2. Malondialdehyde (MDA) Evaluation
As a major product of lipid peroxidation, the level of MDA increased significantly (P < 0.05) in the MDMA group compared to the other groups (Table 1).
4.3. Sperm Parameters
4.3.1. Average Sperm Count
The results showed that the number of sperms decreased in the MDMA group significantly (P < 0.05) compared to the other groups, while the other groups were not significantly different from each other (Table 2).
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | MDMA | MDMA + Vitamin E | Olive Oil | |
| Sperm count, 5 × 105 | 62.5 ± 3.10a | 43 ± 4.3b | 62.5 ± 2.88a | 62.25 ± 1.4a |
| Sperm viability, % | 67 ± 3.1a | 42.25 ± 2.5b | 55.25 ± 3.5c | 68 ± 2.9a |
| Sperm motility, % | 63.25 ± 4.1a | 41 ± 1.8b | 51.25 ± 3.5c | 63.25 ± 5.7a |
| Sperm morphology, % | 82.5 ± 2.8a | 62.5 ± 2.6b | 74 ± 3.5c | 83 ± 4.2a |
aValues are expressed as mean ± SD.
bThrough c represent significant differences between the groups (P < 0.05).
4.3.2. Viability Power
Sperm viability in the MDMA group, compared to the control group, significantly decreased (P < 0.05) in the eosin-nigrosin staining. A highly significant increase (P < 0.05) in sperm viability was observed in the MDMA + Vitamin E group, although there were significant differences (P < 0.05) between the control and MDMA + Vitamin E groups. There was no significant difference in sperm viability between the olive oil group and control group (Table 2 and Figure 1).
4.3.3. Sperm Motility Status
The percentage of motile spermatozoa in the groups showed that there was a significant decrease (P < 0.05) in the MDMA group compared to the control and olive oil groups. Antioxidant administration in the MDMA + Vitamin E group resulted in a significant increase (P < 0.05) in sperm motility in the MDMA + Vitamin E group when compared to the MDMA group, while there was a significant difference (P < 0.05) between the control and MDMA + Vitamin E groups (Table 2).
4.3.4. Sperm Morphology
Sperm with normal morphology appeared in a lower percentage in the MDMA group compared to the other groups (P < 0.05). Vitamin E in the MDMA + Vitamin E group could significantly increase (P < 0.05) this parameter when compared to the MDMA group; however, there was a significant difference (P < 0.05) between the control and MDMA + Vitamin E groups. No significant difference was observed between the control and olive oil groups (Table 2).
4.3.5. DNA Damage
An increase in sperm’s DNA damage was observed in the MDMA group; the increase was significantly highest among the increases in all other groups (P < 0.05). Animals treated with MDMA + Vitamin E showed a significant decrease (P < 0.05) in the mentioned parameter compared to those in the MDMA group, while there was a significant difference (P < 0.05) between the MDMA + Vitamin E and control groups. No significant difference was observed between the control and olive oil groups (Table 3 and Figure 2).
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | MDMA | MDMA + Vitamin E | Olive Oil | |
| DNA integrity of sperms (AO+) | 1.75 ± 0.9a | 10 ± 1.8b | 5.25 ± 1.7c | 1 ± 0.8a |
| Chromatin condensation (AB+) | 0.5 ± 0.5a | 7.5 ± 1.2b | 3.75 ± 0.9c | 0.25 ± 0.5a |
Abbreviations: AB, aniline blue; AO, acridine orange.
aValues are expressed as mean ± SD.
bThrough c show significant (P < 0.05) differences across the groups.
4.3.6. Sperm Nucleus Immaturity
After aniline-blue staining, sperms having high histone in their nuclei with grayish dark blue, based on the histone level, were considered immature sperms. The results indicated a highly significant increase (P < 0.05) in the average percentage of immature sperms in the MDMA group compared to all the other groups. The MDMA + Vitamin E group showed a highly significant decrease (P < 0.05) in this parameter compared to the MDMA group, but there was a significant difference (P < 0.05) between the control and MDMA + Vitamin E groups. The Sperm nucleus immaturity percentage in the olive oil group was similar to that of the control group and no significant difference was observed (Table 3 and Figure 3).
5. Discussion
Results of this study indicated that MDMA treatment significantly increased MDA level in the MDMA group. Nevertheless, with administrating MDMA plus vitamin E in the experimental group 3, MDA level significantly decreased. The damaging effects of active oxygen radicals on spermatogenesis and sperm health were prevented by vitamin E that resulted in testicular oxidative stress (15).
MDMA increases the ROS production and it has been indicated that the increased ROS production makes mitochondrial membranes highly vulnerable to oxidative damage (28). Results of this study showed that vitamin E decreased oxidative stress by increasing the total antioxidant capacity (TAC).
The total sperm number in the MDMA-treated mice turned out to decrease. Hyperthermia has been suggested to occur after MDMA administration (29-31) and it has shown to increase the formation of free radicals (32, 33), which in turn decelerate spermatogenesis finally leading to a reduction in sperm count (33, 34). Our assumption was that MDMA-induced oxidative stress leads to the reduction of sperm number. For our hypothesis to be true, the well-known antioxidant, i.e. vitamin E, should have reversed hazardous effects of MDMA on sperm number. Sperm motility reduced in the MDMA group as compared to the other groups. The remarkable reduction in sperm motility was due to increased ROS content as an outcome of MDMA malperformance that is probably related to ROS generation by MDMA (35, 36). Increased ROS levels considerably affected sperm enzymatic content, and phospholipids peroxidation was increased remarkably by increased ROS levels, which in turn reduced the fluidity of cell membrane and sperm motility, as well (37). However, concerning the preventive effects of vitamin E against ROS and attacks of lipid peroxidation, administration of Vit.E in the MDMA + Vit.E group resulted in a significant enhancement in sperm motility (38). Sperm viability is importantly affected by the sperm plasma membrane integrity and controlled lipid peroxidation. Therefore, serious damage at plasma membrane level increased lipid peroxidation associated with continuing oxidative stress (39). As stated earlier, polyunsaturated fatty acid peroxidation in the sperm is exerted by ROS, which leads to the destruction of sperm mitochondria. This, in turn results in sperm ATP depletion and reduction of sperm viability (40). Our findings accorded to those found by other researchers indicating that Vit.E changed the viability of sperm in the MDMA + Vit.E group significantly. In addition, in comparison with other groups, the number of abnormal sperms in the MDMA group significantly increased. This increase is considered as an indirect result of genotoxicity. Venkatesh et al. showed that there is a significant relationship between reactive oxygen species (ROS) and increase in malformed sperms (41). Based on previous studies, vitamin E can inhibit ROS formation or promote free radical scavenging, so decreased ROS by vitamin E may cause a significant increase in the mean number of normal morphology in the MDMA + Vit.E group compared to the MDMA group (42, 43). Acridine orange (AO) and Aniline-blue (AB) staining determine sperm DNA integrity (double strand DNA versus single strand DNA) (44) and histone-protamine replacement (45).
The results of this study showed that the average number of sperms with damaged DNA in the MDMA group compared to the other groups significantly increased. There are three explanations about the causes of sperm DNA damage: 1) DNA damage is caused by insufficient and inappropriate compaction and twisting of DNA during the period of maturing (46), 2) Oxidative stress results in DNA damage, and 3) Fragmentation of DNA is caused by apoptosis (46). In the MDMA and Vit E groups, nucleotide DNA damage declined due to decreasing oxidative stress and free radicals. The increasing ROS conversely caused changes in all bases, morphological and cross-junction changes of DNA, deletion and uncoupling of complement bases, and changes in reorganization of chromosomes (47). The aniline-blue staining showed a significant increase in the immature sperms percentage (sperms with protamine impairment after MDMA administration). Therefore, it can be concluded that the impact of MDMA is exerted by increasing the DNA disintegrity and affecting DNA packing process. The DNA packing is attributed to the protamine expression rate in early maturation stage (48). Accordingly, MDMA affects the processes of maturation and/or protamine expression, and accordingly the sperm DNA packing is degenerated. Lowered percentage of sperms with positive aniline-blue stained nucleus supported this hypothesis. This study revealed that percentage of immature (low quality chromatin) sperms in the MDMA + Vit.E group compared to MDMA group significantly decreased. The decrease in the mean percentage of spermatozoa with immature nucleus is a strong reason for decreasing the rate of ROS by antioxidant effects of vitamin E. No significant difference between the control and olive groups is in accordance with recent studies; as suggested by Saez Lancellotti et al. administration of olive oil alone did not cause any significant changes in the above-mentioned parameters (49). In conclusion, this study showed that by creating a protection against undesirable effects of MDMA, Vitamin E as an antioxidant increases the quality of sperm in MDMA-treated animals.