1. Background
A growing concern regarding illicit drug use among vulnerable youth has emerged in Iran. National Mental Health Survey data showed that the prevalence of opioid dependence among the 15 to 19-year-old population was 0.96% in 2011 (1). Another household survey in 2011 reported that 13.6% of all 1.325 million drug addicts were 15 to 19-years-old (2). Opium is the most common opioid of use, followed by heroin, crack heroin, and prescription opioids (1, 2). Crack is the street name for a form of the illegal drug in the Iran drug market that its main ingredient is heroin (3). In a national survey among students of 6 to 12th grades in 10 provinces of the country, the prevalence of the lifetime, last year, and last month opium use was 2.5%, 1.5%, and 0.9%, respectively (4).
Vulnerable children, who live or work in streets, are expected to be particularly at risk of drug use disorders. The prevalence of current opioid use is reported in the range of 1.3% to 9% among street children in Iran (5-8). A meta-analysis study showed a high unemployment rate, low socioeconomic status, and illiteracy among parents of street children in Iran. It also indicated that most street children were in contact with their families and came to the streets for work, which suggested the contribution of the low socioeconomic status of the parents to children’s labor in the streets. The weighted prevalence of addiction history among a family member, father, and mother of street children was 56%, 44%, and 23%, respectively (9).
The high prevalence of opioid use among street children necessitates the provision of standard treatment services by specialized drug treatment services within a comprehensive psychosocial support program coordinated by foster care system of the State Welfare Organization (SWO) (10).
There are limited data on safety and effectiveness of opioids agonist medications for treatment of opioids use disorder among children and adolescents (11-13). A few international studies provided data on medication-assisted opioid withdrawal (14-16) and opioid maintenance treatment (16-19) of opioids use disorder among adolescents and youths.
Buprenorphine is safe and effective both in supervised withdrawal and in the maintenance treatment of adult people with opioid use disorder (20). It has been introduced to the network of drug treatment clinics in Iran for the treatment of opioid use disorder since 2006 (21). It is a μ-opioid partial agonist with a safer side effect profile and less severe withdrawal as compared to full opioid agonists (22), which makes it a potential candidate for the treatment of opioids among adolescents (16).
2. Objectives
The aim of this study was to explore the feasibility and effectiveness of inpatient buprenorphine-assisted withdrawal of vulnerable adolescents with opioid use disorder who were referred to the child and adolescent psychiatric ward of Ali Ibn-e-Abi Talib Treatment and Research Center of Zahedan University of Medical Sciences (ZAUMS) in Zahedan, Iran.
3. Patients and Methods
This open-label observational study evaluated the feasibility and effectiveness of buprenorphine-assisted withdrawal for opioid use disorder among vulnerable adolescents due to living or working in streets, who were admitted to a child and adolescent psychiatric ward.
3.1. Setting
The treatment was delivered at the child and adolescent psychiatric ward of Ali Ibn-e-Abi Talib academic general hospital affiliated to Zahedan University of Medical Sciences (ZAUMS), the center of Sistan and Baluchistan province in the southeast of Iran, which provides inpatient and outpatient treatment services under the direct supervision of a child and adolescent psychiatrist. The child and adolescent psychiatric ward staff and equipment were described elsewhere (23).
3.2. Patients
The inclusion criteria were an age of 12 to 17 years, a diagnosis of moderate-to-severe opioid use disorder based on DSM-5, admission to the child and adolescent psychiatric ward, the ability to understand and willingness to provide written assent, and provision of informed consent by parents or legal guardians. The exclusion criteria include having pervasive developmental disorders.
3.3. Intervention
The treatment intervention was part of a program for the management of vulnerable children and adolescents in Zahedan city, in which children and adolescents caught by the police because of living or working in the street were transferred to foster care services since their parents were not competent for guardianship mainly due to active substance use and/or unstable housing.
The vulnerable children and adolescents, who were in need of opioid withdrawal treatment identified by foster care staff, were referred to the hospital with a court order mentioning that they were on the attorney general guardianship and granting consent for necessary treatment interventions. The procedures of buprenorphine-assisted withdrawal were explained to the study participants and their informed assent was obtained. The study was approved by the Ethics Committee of ZAUMS.
In this study, we integrated buprenorphine-assisted opioid withdrawal management within the child and adolescent psychiatric sub-specialty services for adolescents with opioid use disorder. Before that, available services for opioids treatment were limited to the symptomatic management of opioid withdrawal with clonidine, NSAIDs (nonsteroidal anti-inflammatory drugs), and antihistamines, as well as treatment of psychiatric comorbidities (23). We used a flexible dose reduction schedule of buprenorphine-assisted withdrawal regimen (buprenorphine mono-product, 2 mg sublingual tablets manufactured by Faran Shimi Pharmaceutical Co.) from previous studies (16, 24) and trained psychiatric residents for its implementation. Nurses were trained for supervised dosing of buprenorphine in the ward. The clinical psychologist of the ward was also trained to provide needed information regarding rationale, benefits, process, and potential side effects of buprenorphine treatment during an informed assent process to participants. The proper implementation of the treatment plan was supervised by a child and adolescent psychiatrist.
Eligible adolescents, who had a current physiologic dependence to opioids, entered the study. After admission to the ward, a thorough assessment was conducted by psychiatric residents. Patients needed to abstain from opioids at least for six hours for short-acting opioids such as heroin and crack heroin and 8 - 10 hours for long-acting opioids such as opium and opium residues. The first dose of buprenorphine (2 mg) was dispensed by nurses when the patient showed mild-to-moderate withdrawal (> 5 in COWS) measured by the Clinical Opioid Withdrawal Scale (COWS). Since the study participants had been referred from the foster home, there was adequate time passed from their last opioid use and during their first assessment, all of them had moderate to severe withdrawal scores and reported mild to severe pain. The nurses trained patients to hold buprenorphine tablets under their tongues until it dissolved completely. All pharmacotherapies including buprenorphine doses were dispensed under the direct supervision of the nurses. After the first dose of buprenorphine, patients were observed every two hours and other doses of 2 mg buprenorphine (up to 8 mg) were administered if needed. On day 2, patients received the total daily dose of day 1 in the morning. During days 2 and 3, the buprenorphine dose increased to 2 - 4 mg (up to 12 mg) if patients had mild to moderate withdrawal. From day 4, a flexible dose reduction regimen (25% dose reduction every 2 - 3 days) was administered and the buprenorphine dose was tapered and discontinued. The rate of buprenorphine dose reduction was tailored to avoid precipitating withdrawal signs and symptoms. Patients received one buprenorphine dose as their last dose. They tolerated the discontinuation of buprenorphine dose with no need to receive symptomatic medications. All patients also received twice-weekly counseling sessions providing psychoeducation, motivational interviewing, and relapse prevention techniques.
Data were extracted from the patients’ files and analyzed by the statistical package for the social sciences (SPSS), version 20. The treatment staff followed the clinical situation of patients at one and three months after discharge. The study protocol, assessments, and assent process were approved by ZAUMS Ethics Committee.
4. Results
12 adolescents (8 boys and 4 girls) with moderate to severe opioid use disorder were consecutively admitted to the treatment setting, from November 2015 to March 2016. Their age range was 12 to 17 years (mean ± SD: 13.66 ± 1.55). All of the patients were single and did not admit any previous history of sexual activities except two girls (16.6%). Among female patients, no one was pregnant. Regarding educational status, two of them were illiterate and the rest had completed up to seven years of education (average completed years of education: 2.75 ± 2.37). However, at the time of the study, none of them was going to school. Three of the cases did not have any history of education. In terms of their employment status, 6 (50%) of them reported to be vendors, 3 of them reported to be a beggar, and only one of them (8.3%) was a salesman in a store whereas the rest did not report any history of work.
The mothers of all study participants were alive while the fathers of three (33.3%) were deceased. Only the parents of four participants were living together and the parents of the rest were divorced or separated. All adolescents, except one, reported stable housing. The parents of the majority of cases were illiterate. All fathers and 10 (83.3%) mothers suffered from current substance use disorder. According to the court report, none of the families had parenting eligibility because of active drug use of at least one parent (all cases), as well as involvement in drug-related illegal activities mainly selling drugs (case #8). Demographic data of all cases are presented in Table 1.
| Case # | Age, y | Sex | No. of Completed Years of Education | Religion | Work/Source of Income | Paternal Education | Maternal Education | Paternal Current Drug Use | Maternal Current Drug Use | Living Status (Where) | Living Status (with Whom) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | Male | 5 | Shia | Street vend | 8 | 8 | Yes | Yes | Home | Father |
| 2 | 13 | Male | 3 | Shia | Street vend | 0 | 0 | Deceased | Yes | Home | Mother |
| 3 | 15 | Male | 5 | Shia | Street vend | 0 | 0 | Yes | Yes | Home | Father |
| 4 | 17 | Female | 7 | Shia | - | 8 | 5 | Yes | Yes | Home | Mother |
| 5 | 15 | Male | 3 | Shia | Salesclerk | 7 | 0 | Yes | Yes | Home | Mother |
| 6 | 12 | Male | 0 | Sunni | Street vend | 0 | 0 | Yes | Yes | Street | Parents |
| 7 | 14 | Female | 1 | Sunni | - | 0 | 0 | Yes | Yes | Home | Parents |
| 8 | 12 | Male | 1 | Shia | Begging | 0 | 0 | Yes | No | Home | Parents |
| 9 | 13 | Male | 0 | Shia | Street vend | 0 | 0 | Deceased | No | Home | Mother |
| 10 | 12 | Female | 3 | Shia | Begging | 8 | 0 | Yes | Yes | Home | Parents |
| 11 | 15 | Female | 5 | Sunni | Begging | 0 | 0 | Yes | Yes | Home | Mother |
| 12 | 13 | Male | 3 | Shia | Street vend | 0 | 0 | Deceased | Yes | Home | Mother |
The main drug of use by the adolescents was crack heroin while five (41.5%) reported the concurrent use of methamphetamine and four (33.3%) reported the concurrent use of methamphetamine, opium, and or opium sap (shireh). The average cost of using illicit drugs was in the range of 12.000 to 50.000 tomans (mean ± SD: 23.19 ± 10.68). None of the study participants admitted any history of drug injection. Only one (8.3%) youth reported regular cigarette use during the last month. The age at the first use of illicit drugs was between 6 and 14 years old (mean ± SD: 10.17 ± 2.25). None of the cases had any previous history of receiving addiction treatment services. Three boys (cases #1, #3, and #5) reported a previous history of incarceration. Among them, cases #1 and #3 had three and seven months of detention history in juvenile correctional settings, respectively. The drug use history of the cases appears in Table 2.
| Case # | The Main Drug of Use (Last Month) | Concurrent Tobacco Smoking (Last Month) | Use of Other Drugs (Last Month) | Average Cost of Daily Drug Use (Tomans) | History of Injecting Use (Last Month) | Age of the First Illegal Drug Use | Type of the First Illicit Drug | Length of Regular Use of Illicit Drugs (Months) | Involvement in Illegal Activities |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CH | No | MA | 20.000 | No | 12 | OR | 12 | Theft |
| 2 | CH | No | MA | 35.000 | No | 6 | CH, MA | 84 | - |
| 3 | CH | No | MA | 50.000 | No | 11 | CH, MA | 48 | Theft |
| 4 | CH | No | - | 15.000 | No | 14 | CH | 36 | - |
| 5 | CH | No | MA, O, OR | 15.000 | No | 10 | OR | 60 | - |
| 6 | CH | No | MA | 30.000 | No | 12 | CH | 3 | - |
| 7 | CH | No | - | 20.000 | No | 8 | CH, MA | 72 | - |
| 8 | CH | No | MA, O, OR | 12.000 | No | 8 | OR | 48 | Theft |
| 9 | CH | No | MA | 30.000 | No | 8 | CH, MA | 48 | Theft |
| 10 | CH | Yes | MA, O, OR | 20.000 | No | 11 | CH | 10 | - |
| 11 | CH | No | - | 20.000 | No | 11 | CH | 12 | Theft |
| 12 | CH | No | MA, O | 20.000 | No | 11 | CH | 48 | - |
Abbreviations: CH, crack heroin; MA, methamphetamine; O, opium; OR, opium residue (shireh).
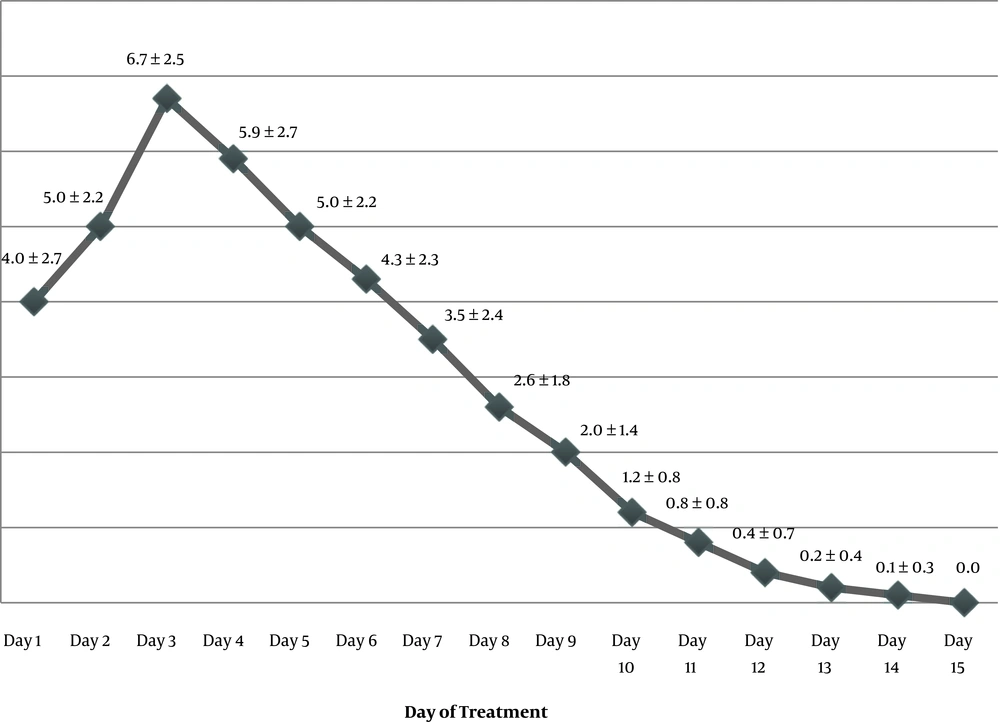
All the adolescents received a medically supervised assisted withdrawal with buprenorphine. The maximum daily dose of buprenorphine was in the range of 2 to 10 mg (mean ± SD: 7 ± 2.6 mg). The duration of buprenorphine dosing was between 5 and 14 days (mean ± SD: 10.4 ± 2.5 mg). Figure 1 presents the average daily dose of buprenorphine from day 1 to day 15. All the complaints of pain and other withdrawal signs and symptoms decreased dramatically within the first four days of treatment. Seven out of eight boys and one out of four girls were diagnosed with pediculosis at the time of hospitalization and they received treatment for it. Three boys (cases 1, 3, and 11) were diagnosed with conduct disorder. One case (#6) suffered from a current diagnosis of PTSD. After discontinuation of the buprenorphine treatment and medical and psychiatric stabilization, the study participants were discharged. The length of the hospital stay was in the range of 12 to 23 days (mean ± SD: 14.66 ± 2.9 days). While most of the cases were discharged within two weeks, the patient with PTSD (case #6) needed a longer duration of inpatient treatment (23 days).
All participants were transferred to the foster care after discharge since none of their parents had eligibility of having their guardianship. At one-month follow-up, all study participants were still in the foster care and the urine tests for morphine and methamphetamine were negative. At the three-month follow-up, the older sister of the only one 15-year-old adolescent (case #3) took the responsibility of his guardianship. The urine tests of all study participants were negative in month 3. Table 3 presents the treatment descriptions of all patients.
| Case # | Length of Buprenorphine Treatment (Days) | Max Buprenorphineose Perayuring Hospitalization (mg) | Length of Hospital Stay (Days) | Retention at One Month | Retention at Three Months |
|---|---|---|---|---|---|
| 1 | 10 | 8 | 14 | Yes | Yes |
| 2 | 12 | 6 | 14 | Yes | Yes |
| 3 | 12 | 8 | 14 | Yes | Yes |
| 4 | 14 | 10 | 15 | Yes | Yes |
| 5 | 11 | 8 | 14 | Yes | Yes |
| 6 | 10 | 4 | 23 | Yes | Yes |
| 7 | 13 | 10 | 17 | Yes | Yes |
| 8 | 11 | 6 | 13 | Yes | Yes |
| 9 | 5 | 4 | 12 | Yes | Yes |
| 10 | 9 | 6 | 13 | Yes | Yes |
| 11 | 7 | 8 | 13 | Yes | Yes |
| 12 | 11 | 6 | 14 | Yes | Yes |
5. Discussion
This study documents the feasibility and effectiveness of inpatient buprenorphine-assisted opioid withdrawal among vulnerable adolescents in Iran. Although the majority of the adolescents who have substance use disorders could receive treatment in outpatient settings (25), the lack of a supportive environment for recovery and high risk for relapse necessitated 24-hour care for all reported cases in this study. This was in line with the international criteria for patient placement in substance use disorder treatment settings (26). Further studies are needed to investigate the safety and efficacy of withdrawal and maintenance treatment with buprenorphine in community settings.
The participants in this study were in the early stages of their adolescence with a mean age of 13.5 years. None of the cases had a previous history of addiction treatment, which might be due to their lack of parental support and supervision, as well as the very low socioeconomic status of their families. This is consistent with international studies mentioning the familial and social factors as potential barriers to treatment access (27, 28). This observation suggests high levels of unmet need for drug treatment among street-connected adolescents with opioid use disorder, which necessitates the implementation of outreach and service linkage programs for them using standard procedures (29).
The main drug of use of all patients was crack heroin and about two-thirds of them reported the concurrent use of other drugs mainly methamphetamine. This drug use profile was comparable with that of participants in a multisite clinical trial of buprenorphine treatment among adolescents (30), but it was in contrast to the review level data on the profile of drug use among street children in resource-constrained countries that reported inhalants as the most common drug of use (31). Besides, it was inconsistent with the national epidemiologic data on the profile of drug use among the 15 to 64-year-old population that showed opium as the main drug of use in the country (1, 32), suggesting more severe and advanced illness among the study participants.
In this study, only two patients reported a history of sexual activities in their lives. This is inconsistent with international studies reporting high rates of risky sexual practices among substance-using street adolescents (33, 34). They also denied any history of injecting drug use in their lifetime. This could be explained by the referral condition of these adolescents from the criminal justice system, as well as the willingness of the study participants to provide socially desirable answers to treatment providers.
Addiction in parents and low social support are the facts found in patients’ history, which are consistent with the results of a previous meta-analysis study in the country (9). Previous studies have documented relatively high rates of attrition and relapse to opioid use following short-term buprenorphine-assisted withdrawal treatment among adolescents (15, 16, 35, 36). Attrition is particularly high among youth who do not receive psychosocial services. Addiction and lack of social stability among parents is associated with higher rates of drug treatment failure among adolescents (28, 37). All these data suggest the high risk for relapse following short-term buprenorphine detoxification among study participants who were street adolescents with a low socioeconomic background. Studies among adults who were dependent on opioids indicated an increased risk of the fatal overdose after discharge from an inpatient detoxification program (38) or correctional settings (39, 40). Therefore, it can be concluded that medically managed opioid withdrawal must be considered only as a part of a comprehensive psychosocial program providing a stable living environment, adolescent welfare, and custody services. In our study, providing a coordinated care by the Child and Adolescent Psychiatric Ward, Provincial Welfare Organization, and Child Custody Court guaranteed the abstinence of patients in a three-month follow-up. Stress resulted from real-life challenges would emerge after returning of the patient to the community, which might happen through granting child custody to a healthy family member or parents after the successful completion of addiction treatment program. Further studies with long-term follow-ups are required to investigate long-term treatment needs and outcomes of vulnerable adolescents with opioid use disorders.
The majority of the patients were discharged after achieving physical and psychiatric stability within 14 days. At the time of the study, none of the child and psychiatric wards provided treatments for opioids use disorder with opioid agonists in the country and this is the first study representing preliminary data on feasibility, safety, and effectiveness of integration of agonist treatment services in child psychiatry settings. However, it should be noted that detoxification is an inadequate treatment for opioids use disorder and it needs to be combined with intensive developmentally appropriate psychosocial support addressing both the adolescents and their families (41). There are limited data that support the extended use of agonist and antagonist medications for treatment of opioid use disorders among adolescents (15-17, 42, 43) although it seems that adolescents with a stable family environment who seek treatment from community settings might be appropriate candidates for extended pharmacotherapies.
Our findings suggest the feasibility of providing inpatient buprenorphine-assisted withdrawal through inpatient sub-child and adolescent psychiatric wards. The treatment program also proved its safety and effectiveness for the management of pain and other opioid withdrawal signs and symptoms among adolescents. Our study has several limitations including observational design, low sample size, and only three months of follow-up.