1. Background
Trichomoniasis is the most common nonviral sexually transmitted infection (STI) worldwide with an estimated incidence of 276 million new cases annually (1, 2). Trichomoniasis is attributed to Trichomonas vaginalis as an anaerobic, flagellated protozoan parasite. Although T. vaginalis affects both men and women, manifestations of trichomoniasis are primarily observed in women (1). In most cases, trichomoniasis tends to be clinically asymptomatic in women and one-third of asymptomatic women become symptomatic within six months (3). The symptoms among women include vaginal discharge (which is often diffuse, malodorous, and yellow-green), dysuria, itching, vulvar irritation, and abdominal pain (3). Trichomoniasis can increase the risk of acquiring or spreading other STIs, as it brings a greater risk of HIV acquisition and transmission (1.5 - 2.7 times) (1) and creates nearly a four-fold increase in HIV shedding (4). Wet mount microscopy is the most commonly used test for detecting T. vaginalis in cervicovaginal specimens, with a sensitivity of 51% to 65% (4). For many years, the culturing technique has been the accepted gold standard for diagnosing T. vaginalis infection, with a sensitivity of 85% - 95% (5). However, this procedure is rather time-consuming and costly. Most importantly, the need for return visits can be a barrier to proper treatment. The sensitivity of the culture method is much less than the sensitivity of nucleic acid amplification tests, including polymerase chain reaction (PCR) (1). However, the determination of T. vaginalis genotypes in asymptomatic or symptomatic patients can represent an image of the epidemiology of the parasite in the region (6, 7).
In some regions of Iran, the prevalence rate of trichomoniasis has been determined between 2% and 8%; the rates can also reach up to 30% depending on the cultural and social status (8-11). So far, no reliable study has been performed on the epidemiology of T. vaginalis in Southeast of Iran.
2. Objectives
This study aimed to represent the first reliable report of T. vaginalis in women with high-risk behaviors in Southeast Iran.
3. Patients and Methods
This study was performed between February and May 2017 to evaluate the molecular epidemiology of T. vaginalis among symptomatic female patients. The patients were selected from a care center for women with high-risk behaviors (defined as risky sexual behaviors, substance abuse, suicidal behaviors, cigarette smoking, alcohol consumption, and other risky behaviors) in Zahedan, Southeast Iran.
Out of 100 women with high-risk behaviors, 90 symptomatic women aged 20 to 60 years were included in the study. Asymptomatic women were excluded from the study. Details on the clinical symptoms, age, education, kind of opiate abuse, and marital status of the patients were obtained from initial request forms. Vaginal swabs and urine specimens were collected in Falcon 15-mL conical centrifuge tubes and plastic cups, respectively. Vaginal specimens were taken with sterile cotton swabs using disposable sterile speculums from the posterior fornix of the vagina (6). The collected samples stored in a plastic box were immediately transferred to the Laboratory of Parasitology, Bu Ali Hospital, Zahedan.
In order to detect T. vaginalis, the vaginal swabs were used for direct observation of wet mount to determine motile trophozoites. The urine specimens were used to inoculate in the diphasic xenic Dorset’s culture medium (12). Then, tube incubation was carried out at 37°C for eight days and the tube was observed microscopically every two days for the presence of trophozoites. Urine specimens were centrifuged (1000×g, 10 min, 4°C) and the sediments were used for the direct observation of wet mount to detect T. vaginalis based on motile trophozoites. All of the positive and negative samples in the microscopic examination were stored at -20°C before DNA extraction and amplification.
To remove the microscopic detection limitation of low sensitivity and to ensure the diagnosis of all the actual positive samples for trophozoites, all the isolates were also DNA extracted and PCR amplified. The sediment related to each sample was washed by phosphate-buffered saline (PBS; pH: 6.4) three times at 8000 rpm for 60 seconds. The DNA extraction was performed using an Exgene stool SV kit (GeneAll Biotechnology Co. Ltd, South Korea) as instructed by the manufacturer (13-15).
The nested PCR-RFLP technique was also used for genotyping by amplification and enzymatic digestion of the actin gene, as previously described (7). The size of the PCR products was assessed by comparing them with a 100 bp-3kb DNA ladder as a size marker (GelRedTM, SMOBiO, Taiwan). PCR amplicons were digested with restriction endonucleases HindII (HincII) (#ER0491, Thermo Scientific, Germany), RsaI (#ER1121, Thermo Scientific, Germany), and MseI (Tru1l) (#ER0981, Thermo Scientific, Germany) (7) and the size of the digested segments was assessed by comparing them with a 50-bp DNA ladder (SinaClon, Iran).
The AccuPrep PCR Purification kit (Bioneer Co., South Korea) was used to purify the PCR products. Then, they were sequenced in both directions using an automated DNA analyzer (Genetic Analyzer 3730; Bioneer Co., South Korea) to examine the size of fragments and confirm the banding patterns. The development of multiple sequence alignments and phylogenetic tree construction were done using the CLUSTAL W program and neighbor-joining (NJ) method in the Kimura's two-parameter model in MEGA version 6.0 (http://www.megasoftware.net/) (16).
In this study, the representative nucleotide sequences and their amino acids sequences were deposited in GenBank (accession no., MF462190 to MF462193).
Microsoft Excel 2010 was used to present the data as spreadsheets. The chi-square test in SPSS version 20 was used for statistical analysis at the significance level of 0.05.
4. Results
Of the 90 isolates from symptomatic patients, 21 (23.3%) were diagnosed to be positive for T. vaginalis based on wet mount microscopy, with the highest prevalence being at the age of 31 to 40 (38.09%) (Table 1). All the wet mount-positive samples were also positive by PCR and cultivation in Dorset medium (Figure 1A). The sensitivity of the wet mount method was similar to the sensitivity of the culture method and PCR. No significant relationship was found between different age groups and trichomoniasis (P > 0.05 on the chi-square test) (Table 1). In terms of marital status, 42.85% (9/21), 28.57% (6/21), 23.8% (5/21), and 4.76% (1/21) of the patients were married, widowed, divorced, and single, respectively (Table 1). In terms of the kind of opiate abuse, 19.4% (4/21), 14.28% (3/21), 57.14% (12/21), and 9.52% (2/21) of the patients were addicted to opium, sap, methamphetamine, and other opiates, respectively (Table 1). In terms of the route of opiate use, 19.4% (4/21) and 80.95% (17/21) of the patients used opiates through ingestion and inhalation, respectively (Table 1). No significant relationship was found between the route of opiate use and trichomoniasis (P > 0.05 on the chi-square test) (Table 1).
| Characteristics | No. of Subjects | Prevalence (%) | P Valuea |
|---|---|---|---|
| Age groups, y | 0.510 | ||
| 20 - 30 | 26 | 5/26 (19.2) | |
| 31 - 40 | 41 | 8/41 (19.5) | |
| 41 - 50 | 15 | 5/15 (33.3) | |
| 51 - 60 | 8 | 3/8 (37.5) | |
| Total | 90 | 21/90 (23.3) | |
| Education level | 0.357 | ||
| Illiterate | 30 | 6/30 (20) | |
| Elementary education | 27 | 10/27 (37) | |
| Intermediate education | 18 | 3/18 (16.7) | |
| Diploma | 10 | 1/10 (10.0) | |
| College education | 5 | 1/5 (20.0) | |
| Total | 90 | 21/90 (23.3) | |
| Marital status | 0.564 | ||
| Single | 8 | 1/8 (12.5) | |
| Divorced | 18 | 5/18 (27.8) | |
| Widowed | 18 | 6/18 (33.3) | |
| Married | 46 | 9/46 (19.6) | |
| Total | 90 | 21/90 (23.3) | |
| Kind of opiate | 0.877 | ||
| Opium | 12 | 4/12 (33.3) | |
| Sap | 16 | 3/16 (18.8) | |
| Methamphetamine (Shisheh) | 54 | 12/54 (22.2) | |
| Other | 8 | 2/8 (25.0) | |
| Total | 90 | 21/90 (23.3) | |
| Opiate use route | 0.738 | ||
| Ingestion | 15 | 4/15 (26.7) | |
| Inhalation | 75 | 17/75 (22.7) | |
| Total | 90 | 21/90 (23.3) |
The Prevalence of T. vaginalis Infection for Women with High-risk Behaviors
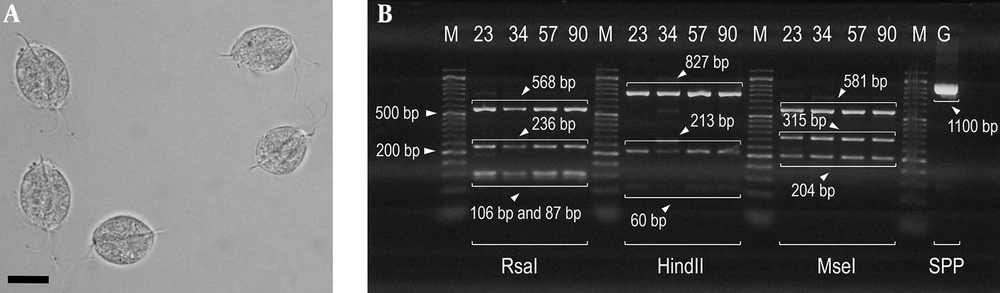
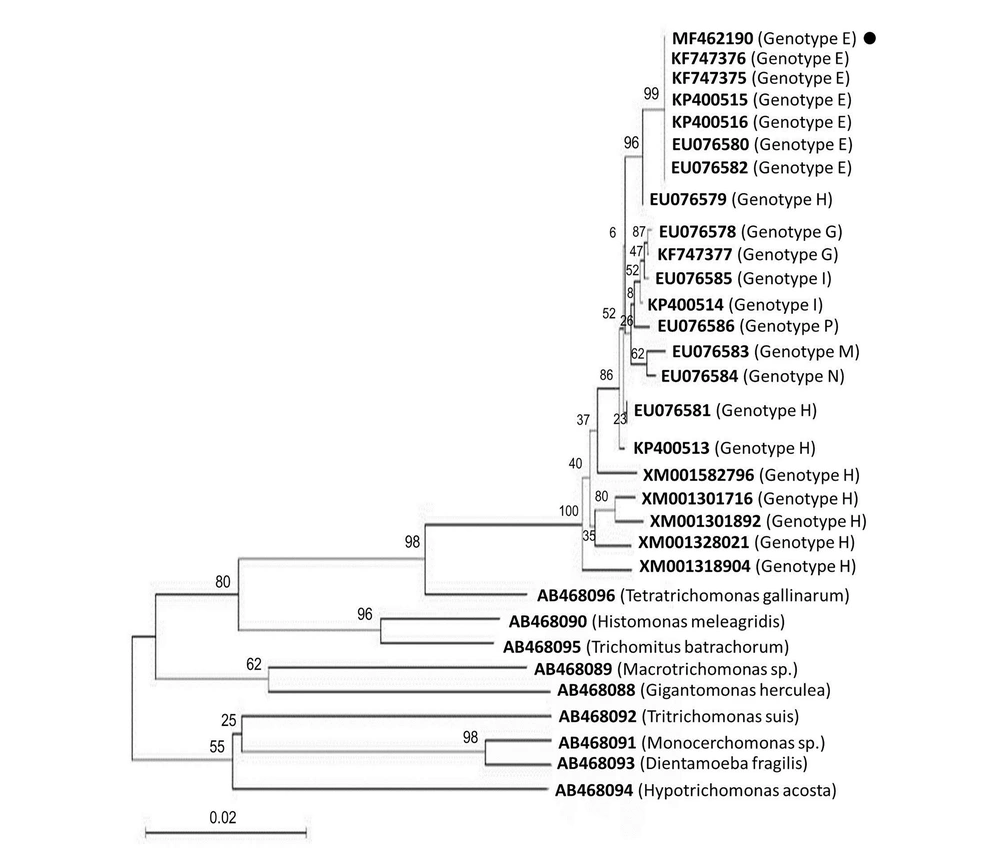
There were only four PCR-positive samples successfully analyzed by RFLP (Figure 1B), and the PCR amplicons (1100 bp) were digested by restriction enzymes RsaI, HindII, and MseI. All of the four samples were related to genotype E. RsaI with the enzyme cutting patterns of 87, 103, 106, 236, and 568 bp showed that these isolates were associated with genotype E (Figure 1B). HindII with the enzyme cutting patterns of 60, 213, 401, 426, and 827 bp displayed that these isolates were related to genotype E (Figure 1B). MseI with the enzyme cutting patterns of 186, 204, 315, 333, 519, and 581 bp indicated that these isolates were associated with genotype E (Figure 1B). Moreover, sequence analysis confirmed that these isolates were defined as genotype E. Representative sequences were compared with some reference partial sequences in the GenBank database (Figure 2). Based on the findings, ≥ 99% homology was detected with T. vaginalis genotype E in the GenBank database for all assemblage E isolates (Figure 2). The nucleotide sequence of 1100 bp has been deposited in GenBank (accession no., MF462190 to MF462193). A phylogenetic tree was constructed based on actin gene sequences for representing the relationships of Trichomonas parasites (Figure 2).
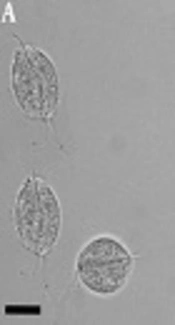
A, T. vaginalis trophozoites detected in urine specimens of symptomatic patients in the current study (bars showing 15 μm); B, Gel electrophoresis of DNA fragments obtained after digesting by PCR-RFLP. Lanes 23, 34, 57, and 90 show the banding patterns after digestion with RsaI, HindII, and MseI (GenBank Accession no. AB468096 and AB4698092); lane M is a 50-bp DNA ladder; lane G (SSP) is the amplified actin gene with size 1100 bp (secondary PCR product).
The phylogeny of T. vaginalis genotypes according to the maximum-likelihood. The phylogenetic tree was constructed based on the multiple alignments of actin gene sequences. Bootstrap values, calculated from 1000 repetitions, are placed at each branch point. Distance represents the number of base substitutions per site. Tetratrichomonas gallinarum and Tritrichomonas suis were considered as outgroup branches. • GenBank accession no. MF462190 is the genotype identified in this study.
5. Discussion
T. vaginalis was detected in 21 out of 90 women with high-risk behaviors included in the study. In the study conducted by Spotin et al. in Tabriz (Northwestern Iran), the rate of infection was determined to be 60.0% (30/50) in asymptomatic women aged > 18 years (6). In Hamadan (Western Iran), the prevalence rate of infection was estimated at 2.1% (16/750) (17). In Ardabil (Northwestern Iran), the rate was 4.48% (41/914) among women aged 18 - 48 years (10). In Kashan (Central Iran), the prevalence among symptomatic women aged 18 - 48 years was reported to be 2.3% (22/970) (18). In Tehran (Northern Iran), the rate of infection was 3.2% (16/500) (8). The global prevalence of trichomoniasis has been estimated between 5% and 74% (19). In the USA, the rate of infection was estimated to be 2.3% in adolescents (20) and 3.1% in women aged 14 - 49 years (21). In Zimbabwe, the prevalence rate of infection in both sexes was calculated to be 9.5% (22). In Ethiopia, the rate was 6.3% among pregnant women (23). In Zambia, the rate of infection was evaluated to be 32.2% in pregnant women and 24.6% in adolescent girls (7). In Turkey, the rate of infection was 9% in women aged 18 - 45 years (24). The results of these studies are not strictly comparable since evaluations were done using different detection procedures. In addition, differences may be attributed to the risk factor profile of the population and the geographical locations.
In the present study, the sensitivity of the wet mount and culture methods was equal. In previous studies, such as the study conducted by Ahady et al. (10), the sensitivity of the culture method was reported to be higher than the sensitivity of wet mount.
Surprisingly, molecular amplification did not detect more T. vaginalis cases than the wet mount or culture methods did. Indeed, the three methods performed equally well. It was expected that the molecular method would produce better results; however, this weakness may be attributed to the low amount of amplified DNA for visualization (13-15).
In the current study, genotype E of T. vaginalis was identified by RFLP analysis. The results reported in our study correspond well with those from studies published in Iran by other researchers. The findings of the present investigation are supported by a study that comprehensively accomplished to identify the different genotypes of T. vaginalis in symptomatic patients based on the RFLP method of the actin gene (7). In the study conducted by Momeni et al. (9) in Karaj (Northern Iran), 45 microscopy-positive samples were examined via PCR-RFLP. The findings showed that 23 (51.1%), 11 (24.4%), 6 (13.3%), and 3 (6.6%) samples contained genotype G, genotype E, genotype H, and genotype I, respectively. Similarly, an Iranian study in Tabriz (Northwestern Iran) that examined 50 suspected clinical samples found that 73.4% (20/30) were genotype G and 26.6% (8/30) were genotype E (6). In our study, all isolates were related to genotype E. However, it can be easily understood that the non-observance of individual health has caused genotype E to be scattered in women referring to our care center.
There is no published report of the distribution of T. vaginalis in women in the southeast of Iran. The present study provides information about the distribution of T. vaginalis in southeast Iran. However, further studies with more clinical samples may increase our knowledge about the distribution of this parasite in Sistan and Baluchistan province.