1. Background
Human T-lymphotropic virus 1 (HTLV-1) is found primarily in persons originating from or having sexual contact with individuals from endemic areas, such as Japan or the Caribbean basin. Sexual transmission of HTLV-1 has been widely reported in these individuals and other populations, including the United States and West Africa. Some research indicates that HTLV-1 may be transmitted more efficiently from males to females than vice versa (1). The HTLV-1 virus, discovered in 1980, is associated with human T cell lymphoma (ATLL), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (2, 3). The association of psychiatric problems with viral infections has always been taken into consideration. Similar to HIV and hepatitis C infections, the symptoms of depression and anxiety, as well as different aspects of lifestyle, have been investigated (4, 5). However, there are limited numbers of studies and data on psychiatric problems associated with HTLV-1 infection. These few studies also indicate a high level of depression and anxiety symptoms, and subsequently, impaired lifestyle in patients with HTLV-1 (6-9).
Awareness of having chronic infection is per se a harmful and stressful experience such that the blood donors diagnosed with HTLV-1 experienced a great number of mental disturbances, which was associated with negative mental and social impacts (10-12). Moreover, physical disabilities and the associated limitations in daily and professional activities, as well as social isolation, may justify the high prevalence of mental disorders in HAM/TSP patients (6, 13).
This is an endemic virus in some places throughout the world, including Japan, Brazil, North Palestine, and the northeast part of Iran (14, 15). The prevalence of this virus in Mashhad (the second metropolis of Iran) and among those moved from this city to other places is specifically high such that a seroepidemiological study in Mashhad estimated its prevalence at 2.12% (15).
According to the above studies, the prevalence of psychiatric disorders in such patients (either the healthy carriers or HAM/TSP patients) may be higher than the general population. However, how the HTLV-1 affects the psychological performance, and the prevalence of psychiatric manifestations is unknown in these patients, especially in Khurasan (in the northeast of Iran) as an endemic area.
2. Objectives
Owing to a relatively high prevalence of this disease and viral infection in Mashhad (the capital city of Khurasan Province), this study aimed at estimating the frequency of psychiatric disorders in HAM/TSP patients compared to the healthy carriers and healthy non-carrier individuals to take required measures for the referral and treatment of such patients.
3. Patients and Methods
This cross-sectional study included three groups selected from individuals visiting the Neurological Clinic of the Qhaem Hospital of Mashhad from September 23, 2013, to February 20, 2015: (1) Those with HAM/TSP diagnosed by a neurologist, and based on inclusion criteria; (2) asymptomatic individuals with positive serology for HTLV-1; and (3) non-infected individuals. The third group was referred by the virus reference laboratory and matched with the carrier group in terms of background variables (age and gender). All subjects were screened using SCL90 inventory for psychiatric symptoms. Mirzaee and Rezapour’s research results indicate good internal concurrent validity and reliability test-retest method in the Iranian population, and the sensitivity of this instrument is reported by about 90% (16). Then the subjects who scored higher than the cut-off point in each screening evaluation attended structured clinical interviews supervised by two psychiatrists. The diagnosis was based on the fourth edition of the diagnostic and statistical manual of mental disorders (DSM-IV-TR). The results obtained from these three groups were analyzed.
Subjects were included into the study after the confirmation of HTLV-1 antibodies in serum (with Western Blot tests), the absence of any clinical sign or syndrome in asymptomatic HTLV-1 group, the presence of HTLV-1 antibodies in serum and CSF in HAM/TSP group, and the assessment of the level of motor-disability by a neurologist, based on Osame’s motor disability score (OMDS).
The exclusion criteria were mental retardation, positive serum result for other blood-transmitted viruses (HIV, HBV, and HCV), infection with chronic medical disease, infection with epilepsy and other neurological problems, a history of traumatic brain injury, pharmacotherapy with corticosteroids and/or interferon in myelopathy group, hospitalization for a positive history of major psychiatric disorders, the use of psychotropic drugs by the subject or one of the first-degree relatives, a history of a serious stressor (loss of first-degree relatives, divorce, loss of job, immigration, and serious emotional breakdown) in the past three months. It should be noted that the study was approved by the Ethics Committee of Mashhad University of Medical Sciences (approved code: 910385).
3.1. Statistical Analysis
To compare the groups in terms of quantitative variables, one-way analysis of variance (ANOVA) and/or the Kruskal-Wallis test were used after controlling the normality of variables. The results were analyzed with descriptive statistical methods, the Mann-Whitney test, the Two-Sample Kolmogorov-Smirnov test, and the spearman correlation test. Qualitative variables were assessed with the chi-square test, and SPSS19 was used for statistical analysis. Findings were considered significant at P < 0.05.
4. Results
In general, 116 patients were studied (30 subjects in HAM/TSP group, 45 subjects in asymptomatic group (carriers), and 41 HTLV-1 seronegative individuals in the control group) (Table 1).
| Variables | HAM/TSP | Carrier | Control |
|---|---|---|---|
| Age | 49 ± 9.9 | 39.36 ± 8.30 | 41.61 ± 11.40 |
| Sex | |||
| Female | 25 (83.3) | 15 (33.3) | 23 (56.1) |
| Male | 5 (16.7) | 30 (66.7) | 18 (43.9) |
| Marital status | |||
| Married | 22 (73.3) | 43 (95.6) | 34 (82.9) |
| Single | 5 (16.7) | 2 (4.4) | 2 (4.9) |
| Divorced | 3 (10.0) | - | 5 (12.2) |
| Occupation | |||
| Active | 8 (26.7) | 37 (82.2) | 29 (70.7) |
| Unemployed | 22 (73.3) | 8 (17.8) | 12 (29.2) |
Sociodemographic Characteristics of Patientsa
In terms of matching the groups, due to sex and age differences in the myelopathy and carrier groups, samples in the healthy group were matched with the carrier ones. The independent t-test showed no significant difference between the two groups with respect to gender and age variables (P = 0.1 and V = 3.63 in terms of gender, and P = 0.09 and F = 3.762 in terms of age). With respect to the economic status variable, the three groups were distributed similarly (P = 0.07).
It should be noted that because the carriers of the virus that developed spastic paraparesis had a higher age and were often female, the matching of the three groups was not possible. Because one of the main goals of the study was to evaluate the direct effect of the virus on the brain, the control group was matched with the carrier group.
Global severity index (GSI) is a composite grading index that measures experienced stress, along with some reported symptoms. It is the best single distress scale and should be used when a single scale suffices. The general rule is that the T score of more than 63 indicates the presence of a significant level of psychological problems (17). The results from statistical analysis with Fisher’s Exact test showed a significant difference between the carrier and HAM/TSP groups in this regard. In fact, five subjects (16.5%), all from the HAM/TSP group, obtained GSI > 63 (P = 0.008).
The structured interview-based comparison of frequency distribution of psychiatric problems showed that the prevalence of major depression among the HAM/TSP and carrier groups was significantly different (based on the chi-square test). According to the results, nine infected subjects (75%) were from the HAM/TSP group. The comparison of the frequency distribution of dysthymic disorder (based on Fisher’s exact test) showed no significant difference between the HAM/TSP and carrier groups (P = 0.150, value = 3.571). A comparison of the frequency distribution of the major depressive disorder with the Chi-square test showed a significant difference between the HAM/TSP and control groups (P = 0.011, value = 6.542). Fisher’s Exact test showed a significant difference between HAM/TSP and control group in terms of dysthymic disorder (P = 0.028, value = 5.793). It is worth noting that the comparison of frequency distribution of the major depressive disorder (P = 0.603, value = 0.449) and dysthymic disorder (P = 1, value = 0.922), using Fisher’s Exact test, showed no significant difference between the carrier and control groups. A comparison of the HAM/TSP group with the carrier group, using the chi-square test, showed no significant difference in the frequency distribution of generalized anxiety (P = 0.141, value = 2.170). This difference was not significant between the HAM/TSP group and the control group, too (P = 0.062, value = 3.490). According to Table 2, the comparison of the frequency distribution of this disorder also showed no significant difference between the carrier and control groups (P = 0.857, value = 0.032).
| Disorder | Overall Frequencies | HAM/TSP | Carriers | Controls |
|---|---|---|---|---|
| Major depressive disorder | 12 (10.3) | 9 (75) | 1 (8.3) | 2 (16.7) |
| Dysthymic disorder | 5 (4.3) | 4 (80) | 1 (20) | 0 (0) |
| Depressive disorder not otherwise specified | 10 (8.6) | 3 (30) | 4 (40) | 3 (30) |
| Generalized anxiety disorder | 19 (16.4) | 9 (47.4) | 6 (31.6) | 4 (21.1) |
| Phobic disorder | 8 (6.9) | 4 (50) | 1 (12.5) | 3 (37.5) |
| Obsessive-compulsive disorder | 7 (6) | 3 (42.9) | 2 (28.6) | 2 (28.6) |
| Panic disorder | 2 (1.7) | 1 (50) | 0 (0) | 1 (50) |
| Anxiety disorder not otherwise specified | 11 (9.5) | 5 (45.5) | 3 (27.3) | 3 (27.3) |
Frequency Distribution of the Development of Psychiatric Disorders Based on DSM-IV-TRa
The investigation into the median of ranks based on Osame’s score in patients with a major depressive disorder and generalized anxiety disorder as well as patients without these disorders in the HAM/TSP group, based on the non-parametric Mann-Whitney U-test, revealed the following findings: at the level of the major depressive disorder, the median of ranks was higher in patients with a major depressive disorder compared with mentally stable patients. In fact, patients with major depression obtained a higher score in terms of the severity of the motor disability. This increase of median scores in the major depression group was positive and significant (P < 0.001).
(Mean rank = 25.44, median = 1, interquartile range (IQR) = 1 - 1.5 in major depressive disorder group; mean rank = 11.24, median = 2, IQR = 1 - 3 in non-major depressive disorder group).
At the level of generalized anxiety disorder (GAD), the Mann-Whitney U-test showed more severe motor disability in HAM/TSP patients with GAD, based on the Osame’s score. In fact, the patients with an anxiety disorder had a significantly higher median score in grading (P = 0.018).
(Mean rank = 21.11, median = 1, IQR = 1 - 1.5 in generalized depressive disorder group; mean rank = 13.10, median = 2, IQR = 1 - 3 in non-generalized depressive disorder group).
The investigation into the Osame’s score-based median of ranks in patients with GSI > 63 and patients with GSI < 63 in the HAM/TSP group, using two-sample Kolmogorov-Smirnov test, showed a significant difference in this score between the two groups. The median score was higher in the HAM/TSP group with GSI > 63 (median = 5, IQR = 4.5 - 5.5). On the other hand, the median score was lower in the HAM/TSP group with GSI < 63 (median = 3 and IRQ = 2 - 3.5). In effect, patients who experienced a significant level of mental distress (based on SCL-90) obtained a higher score in motor disability level.
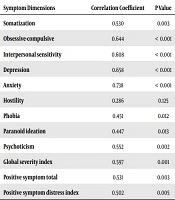
The relationship assessment of psychiatric dimension scores of SCL-90 with the severity of neurological symptoms, based on Osame’s score and using the Spearman test showed a positive significant correlation of grading, based on the Osame’s score, with the scores of somatization, obsession, interpersonal sensitivity, depression, anxiety, phobias, paranoid ideation, and psychoticism, and also between the grade of OMDS and the scores of GSI, PSDI, and PST. These significant correlations existed even after controlling the age as a confounding variable (Table 3).
| Symptom Dimensions | Correlation Coefficient | P Value |
|---|---|---|
| Somatization | 0.530 | 0.003 |
| Obsessive-compulsive | 0.644 | < 0.001 |
| Interpersonal sensitivity | 0.608 | < 0.001 |
| Depression | 0.658 | < 0.001 |
| Anxiety | 0.738 | < 0.001 |
| Hostility | 0.286 | 0.125 |
| Phobia | 0.451 | 0.012 |
| Paranoid ideation | 0.447 | 0.013 |
| Psychoticism | 0.552 | 0.002 |
| Global severity index | 0.597 | 0.001 |
| Positive symptom total | 0.531 | 0.003 |
| Positive symptom distress index | 0.502 | 0.005 |
Relationship of Psychiatric Dimension Scores of SCL-90 with the Severity of Neurological Symptoms, Based on Osame’s Score
5. Discussion
In the current study, the overall frequency of the major depressive disorder in the HAM/TSP group was estimated at 30%, which was higher than the overall prevalence of this problem among the general population (17%) (18). In a study in Brazil, Boa-Sorte et al. (13) reported the prevalence of major depressive disorder among seropositive individuals as 37.96%. Although they did not report the prevalence of this problem in the HAM/TSP group separately (13). The current study showed a significant difference between the symptomatic and asymptomatic groups in the frequency of major depression (P = 0.002). This difference was also significant in frequency comparison of this disorder between the symptomatic and seronegative groups (P = 0.011), whereas there was no significant difference between the carrier and seronegative groups (P = 0.603). In a study by Stumpf et al. (7), the prevalence of depression was significantly higher among asymptomatic carriers than the seronegative group. This finding was inconsistent with our findings. This difference may be due to the lack of a unique diagnostic instrument, and different genetic and sociocultural backgrounds. Moreover, individuals under treatment with antidepressants, steroids, and interferon were excluded from our study. Our findings were consistent with the findings of Gascon et al. (19), in which a significantly higher prevalence of depression was reported in symptomatic patients.
Different hypotheses have been proposed on the etiology of depression among myelopathy patients. According to a hypothesis, there is a probable correlation between viral-induced depression with increased production of pro-inflammatory cytokines such as interleukin-1, tumor necrosis factor (TNF-α), and interleukin-6 (6, 8). On the other hand, Guiltinan e al. (20) attributed the higher prevalence of depression in developing countries such as Brazil to cultural and socioeconomic aspects. In addition, regarding that the prevalence of depression has been reported higher in the HAM/TSP group by different studies, this finding may be due to the psychological burden of clinical symptoms of disability and social isolation-induced by myelopathy (6, 13).
The investigation into frequency distribution of psychiatric disorders based on the severity of neurological motor symptoms in the myelopathy group, according to the Osame’s score criterion, showed that the development of the major depressive disorder in HAM/TSP patients was significantly correlated with the severity of neurological motor symptoms (P = 0.001). It is worth noting that the highest frequency distribution of patients with a major depressive disorder was at scores 4 and 5. It should also be noted that the patients with myelopathy (grade 4) were in need of others’ help. In fact, patients with a grading score below four could walk without help. The need for assistance initiated in grade 4 for walking up- or downstairs. Parallel with these findings, the current study showed a higher frequency distribution of milder depressive syndromes (i.e., dysthymic disorder and depressive disorder not otherwise specified) among patients with grading scores of 2 and 3. Poor social relations and physical intimacy behaviors, along with social isolation, which results in a higher prevalence of depressive disorder in other chronic physical diseases (21), were also observed in this illness. Although the exact correlation of myelopathy development with the prevalence of depression is unknown, the expansion of psychiatric services and mental support is recommended for patients at risk of depressive disorders. It is also recommended to perform further studies on this field and include mental evaluation of patients in treatment protocols.
Statistical analysis in the investigation into the correlation of scores in the checklist of disease symptoms with the severity of neurological signs (graded based on the Osame’s score) showed a significant correlation between the symptoms and severity of myelopathy in patients. In fact, increased myelopathy scores of the checklist of symptoms at eight dimensions’ levels, namely somatization, obsession, interpersonal sensitivity, depression, anxiety, phobias, paranoid ideation, and psychoticism, as well as GSI, PSDI, and PST indices, increased the scores of the checklist’s symptoms. In addition, this correlation remained positive and significant even after the removal of a probable confounding variable, i.e., age. This relationship was not observed between hostility and Osame’s score. According to the self-reporting questionnaire, there was no significant relationship between the neuropathy severity and intensity of hostility (even after controlling the age variable). This finding indicates vast experiences of mental symptoms, which per se can affect the quality of life of patients. In other words, in addition to physical problems, patients suffer from several mental symptoms that may make disease flow and consequences more complicated.
The results showed that patients with a major depressive and generalized anxiety disorder obtained higher grading scores in the severity of myelopathy. In fact, similar to chronic physical diseases, increased severity of the disease resulted in increased physical disabilities, loss of physical independence and control over the disease, social isolation, and increased frequency of major depression and generalized anxiety disorders. The development of psychiatric disorders per se negatively affects the flow of underlying medical diseases (such as myelopathy).
In this study, the overall frequency of depression and anxiety disorders was about 30%, which was almost similar in patients with chronic physical diseases (21% - 35%) (21). Regarding an insignificant difference of the frequency of the major depressive and generalized anxiety disorders in the seronegative and carrier groups, but a significant increase in the frequency of these two disorders in the myelopathy group, as well as a higher frequency with myelopathy increase, it seems that the presence of a chronic physical illness is a riskier factor than HTLV-1 itself, as a primary cause of a psychiatric disorder for an increased prevalence of anxiety and depression among myelopathy patients. This finding is consistent with the findings of Carvalho et al. (6) in Brazil. Authors of this article believe that the development of mental illnesses is probably the consequence of critical deficiencies caused by this debilitating disease (6). Another study by Gascon et al. (19) showed that patients experienced significantly higher degrees of depression, anxiety, and impairment of quality of life than asymptomatic carriers. In addition, in this study, the existence of myelopathy and the onset of clinical symptoms significantly correlated with the prevalence of depression. The provision of psychiatric treatments, along with the improvement of psychological supports and the establishment of social networks for such patients may improve their compliance with disabilities and the quality of life, which can be a subject for future studies.
5.1. Conclusions
This was the first research on the relationship of frequency of symptoms and psychiatric disorders in myelopathy associated spastic paresis patients in the HTLV-1 carrier group in Mashhad, as an endemic region. It is recommended to take appropriate measures to provide psychiatric consults in related clinics for prevention, periodical evaluations, and treatment of psychiatric disorders. Finally, since patients’ quality of life, medical service provision, and exploitation of such services by patients can be improved by treating depression, and regarding the sensitivity of patients to mental disorders, a psychiatric evaluation of patients is recommended as a component of interventional protocols.
5.2. Limitations
This study had several limitations, including small sample size and more women in the myelopathy group, which might affect the frequency distribution of the disorders. So it is suggested to be considered in future studies.