1. Background
Fetuin-A, is a glycoprotein which is known as a biomarker for diabetes risk (1, 2). It seems that high levels of fetuin-A can be related to insulin resistance (IR) (3, 4). A relationship has been observed between high fetuin-A level and impaired glucose tolerance, metabolic syndrome and lipid profile (5, 6). IR is important in pathophysiological mechanisms of T2DM, which may contribute to the development of dyslipidemia, hypertension and vascular complications (7, 8). Adipsin is the major protein of adipose cells. It is paradoxically reduced in many animal models of obesity and diabetes which are known to be the complement factor D (CFD) (9, 10). It catalyzes the rate-limiting step of the alternative pathway of complement activation (11, 12). Adipsin mRNA abundance reduces during the glucose infusion, which makes a hyperglycemic, hyperinsulinemic status (9). It is reported that adipsin is useful in maintaining β cell function (12, 13). There is association between obesity, adipose inflammation and malfunction of β cells (13). Nevertheless, the role of fetuin-A and adipsin in patients with T2DM has not been fully studied.
2. Objectives
This study aimed to investigate the concentration of fetuin-A and adipsin in T2DM patients and healthy subjects. Besides, their relationship with lipid profile, and biochemical factors were studied.
3. Patients and Methods
In this case-control study, 43 subjects; 13 males and 30 females (mean age 58.5 ± 9.5 years) referred at Diabetes Clinic Center of Ali-Asghar, a general hospital in Zahedan University of Medical Science (ZAUMS), Iran. All patients were enrolled during the period of November to December 2017. They referred to the diabetes clinic center for follow up of treatment regularly.
The clinical criteria for diabetes of patients were approved by an endocrinologist regularly. According to the definition of World Health Organization (WHO), criteria for diagnosing diabetes include fasting blood sugar (FBS) level equal to or greater than 126 mg/dL, 2-hour oral glucose tolerance test (OGTT) result equal to or greater than 200 mg/dL, and hemoglobin A1c equal to 6.5% or higher. T2DM was medically confirmed by a specialist physician. The patients were monthly referred to a diabetes clinic for treatment and health care. All patients completed the study without any problem. In control group, 41 healthy subjects including 18 males and 23 females (mean age 57.5 ± 11.3 years) were selected which matched the case group regarding sex and age. The control group included healthy persons with no medical histories (diabetes, hypertension, heart disease, renal and liver disease malignancy, endocrine disorders) and/or addiction. Exclusion criteria for both groups were; BMI ≥ 40 kg/m2, age less than 18 years and absence of any systemic disease. All eligible patients had a history of T2DM as follows: 14 patients (32.6%) less than 6 months, 18 patients (41.9%) between 6 months to one year, and 11 patients (25.6%) more than two years. They were treated by oral glucose-lowering medications. The ZAUMS board of ethics approved the study protocol (Code number; No. 7480; 25, October 2017). So, all aims of the protocol were clearly elucidated to the subjects.
3.1. Anthropometric and General Characteristics Measurements
The gender, age, height, and body weight, of participants were recorded. Weight and height were measured while subjects were wearing light clothes and with bare feet, using a Detector scale with the accuracy of 0.1 kg for weight and 0.5 cm for height. Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared.
3.2. Blood Sampling and Determination of Lipid Profile
After 10 - 12 hours overnight fasting, blood samples were collected at 8:00 a.m. from the two groups. Afterwards, blood samples were centrifuged at 1500 x g for 10 minutes. Total cholesterol, LDL-C, HDL-C and triglyceride were measured by applying a colorimetric method using Autoanalyser RA-1000 (Pars Azmoon kits, Tehran, Iran), along with HbA1c, in all subjects. Glucose (FBS), blood urea nitrogen (BUN), creatinine, and uric acid levels of the participants were also determined using photometric method in participants. The remained samples were stored at -70°C for further analysis.
3.3. Measurement of Plasma Fetuin-A Levels and Adipsin (CFD)
Serum levels of fetuin-A were measured using enzyme- linked immune-sorbent assay (ELISA) by commercial kits: Human Fetuin-A (FETU-A) ELISA kit (Cat. No. CK-E11353; Hangzhou Eastbiopharm Co., LTD). Serum adipsin (Human Complement Factor D) was measured by using Enzyme-Linked Immune-sorbent Assay (ELISA Kit) (Cat. No. CK-E91447; Hangzhou Eastbiopharm Co., LTD). The intra assay coefficient of variation (CV) of both adipokine including; fetuin -A and adipsin (Complement factor D) ranged from 10% to 12%.
3.4. Statistical Analysis
All data are presented as mean ± standard deviation. Comparisons between two groups were analyzed by t-test or Mann-Whitney U-test. Pearson and Spearman correlation coefficients were used for assessment of parametric and non-parametric correlations respectively. All statistical analysis was performed using SPSS software (version 18 for Windows, Chicago, USA). Data were considered significant at a level of P < 0.05.
4. Results
The results (Man-Whitney U-test) showed that there was a significant difference between mean levels of fetuin-A (P = 0.03), and adipsin (CFD) (P = 0.001) in the two groups. There was a significant positive correlation between fetuin-A and adipsin in case (r = 0.94, P < 0.0001), and control (r = 0.96, P < 0.0001) groups. In serum of male subjects in case group, fetuin concentration was 617.5 ± 644.1 mg/dL and adipsin (CFD) was 12.9 ± 10.5 mg/dL. But in female subjects of case group, fetuin-A and adipsin (CFD) concentrations were 631.4 ± 661.9 mg/L and 13.0 ± 12.4, respectively. There was no significant difference based on other variables between male and female in case group (Table 1).
| Groups | Variables | |
|---|---|---|
| Fetuin-A (mg/dL) | Adipsin (CFD) (mg/dL) | |
| Case | ||
| Male | 617.5 ± 644.1 (117.7 - 1966) | 12.9 ± 10.5 (4.1 - 32.9) |
| Female | 631.4 ± 661.9 (170.6 - 2480) | 13.0 ± 12.4 (3.3 - 51.5) |
| Total | 627.2 ± 649.0 (117.7 - 2480.9) | 13 ± 11.7 (4.1 - 51.5) |
| Control | ||
| Male | 960.6 ± 819.6 (297.9 - 2593) | 20.5 ± 14.6 (8 - 55) |
| Female | 661.7 ± 615.9 (163.2 - 2630) | 16.07 ± 12.4 (2 - 47.6) |
| Total | 789.8 ± 716.7 (163 - 2630) | 18.0 ± 13.4 (2 - 55) |
aValues are expressed as mean ± SD and (range).
There was not a significant difference, based on general characteristics, between the two groups (Table 2). The mean level of HbA1C in diabetic patients was found as follows: male (8.8 ± 1.4%), female (9.1 ± 1.5%), and totally (9.0 ± 1.5%). There was also a significant correlation between F.B.S and HbA1C in diabetic patients (r = 0.35, P = 0.02).
| Indicators | Groups | P Value | |||||
|---|---|---|---|---|---|---|---|
| Case (N = 43) (51.1%) | Control (N = 41) (48.9%) | ||||||
| Male (N = 13) | Female (N = 30) | Total | Male (N = 18) | Female (N = 23) | Total | ||
| Age, y | 55.54 ± 6.25 (40 - 63) | 55.9 ± 10.04 (36 - 76) | 55.84 ± 8.98 (36 - 81) | 52.61 ± 7.75 (40 - 74) | 52.6 ± 7.7 (40 - 76) | 52.52 ± 7.62 (40 - 76) | 0.07 |
| Weight, kg | 78.9 ± 14.3 (65 - 110) | 71.5 ± 4.8 (45 - 100) | 73.72 ± 14.9 (45 - 100) | 73.94 ± 11.8 (45 - 92) | 73.58 ± 16.6 (45 - 110) | 73.74 ± 14.5 (45 - 110) | 0.9 |
| Height, cm | 168.5 ± 5.7 (155 - 177) | 158 ± 6.1 (148 - 175) | 161.2 ± 7.6 (148 - 177) | 162.8 ± 7.3 (155 - 175) | 161.5 ± 7.8 (150 - 177) | 162.1 ± 7.54 (155 - 177) | 0.5 |
| BMI, kg/m2 | 27.2 ± 3.7 (22 - 35) | 28.5 ± 5.9 (18 - 44) | 28.1 ± 5.4 (18 - 44) | 28.6 ± 4.4 (21 - 37) | 28 ± 6 (18 - 44) | 28.3 ± 5.3 (18 - 44) | 0.8 |
aValues are expressed as mean ± SD and (range).
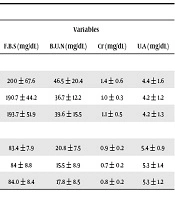
No significant difference was observed between duration of T2DM and gender in study group (P = 0.8) (Table 3).The results also showed that there was a significant difference between the two groups with regard to mean of FBS (P < 0.001), blood urea nitrogen (BUN) (P < 0.001), creatinine (P = 0.001), and uric acid (UA) (P = 0.001) levels (Table 4).
| Sex | Duration of Diabetes | |||
|---|---|---|---|---|
| Less than 6 months | Between 6 Months to 1 Year | Between 1 to 2 Years | Total | |
| Male | 5 (38.5) | 5 (38.5) | 3 (23.1) | 13 (100) |
| Female | 9 (30) | 13 (43.3) | 8 (26.7) | 30 (100) |
| Total | 14 (32.6) | 18 (41.9) | 11 (25.5) | 43 (100) |
aValues are expressed as No. (%).
| Groups | Variables | |||
|---|---|---|---|---|
| F.B.S (mg/dL) | B.U.N (mg/dL) | Cr (mg/dL) | U.A (mg/dL) | |
| Case | ||||
| Male | 200 ± 67.6 | 46.5 ± 20.4 | 1.4 ± 0.6 | 4.4 ± 1.6 |
| Female | 190.7 ± 44.2 | 36.7 ± 12.2 | 1.0 ± 0.3 | 4.2 ± 1.2 |
| Total | 193.7 ± 51.9 | 39.6 ± 15.5 | 1.1 ± 0.5 | 4.2 ± 1.3 |
| Control | ||||
| Male | 83.4 ± 7.9 | 20.8 ± 7.5 | 0.9 ± 0.2 | 5.4 ± 0.9 |
| Female | 84 ± 8.8 | 15.5 ± 8.9 | 0.7 ± 0.2 | 5.3 ± 1.4 |
| Total | 84.0 ± 8.4 | 17.8 ± 8.5 | 0.8 ± 0.2 | 5.3 ± 1.2 |
Abbreviations: B.U.N, blood urea nitrogen; Cr, creatinine; F.B.S, fasting blood sugar; U.A, uric acid.
aValues are expressed as mean ± SD.
The results also showed that there was only significant difference between the two groups regarding mean of triglyceride level (P < 0.001). However, no significant difference was observed between the two groups with regard to mean of cholesterol (P = 0.3), LDL (P = 0.1), and HDL (P = 0.5) levels (Table 5).
| Groups | Variables | |||
|---|---|---|---|---|
| Cholesterol (mg/dL) | Triglyceride (mg/dL) | LDL (mg/dL) | HDL (mg/dL) | |
| Case | ||||
| Male | 156.7 ± 42.2 | 177.0 ± 77.4 | 77.4 ± 49.1 | 44.9 ± 8.8 |
| Female | 164 ± 36.9 | 167.4 ± 80.9 | 79.9 ± 22.9 | 53.8 ± 15.4 |
| Total | 161.7 ± 38.3 | 170.4 ± 79.0 | 79.1 ± 35.7 | 51.3 ± 14.3 |
| Control | ||||
| Male | 143.7 ± 38.9 | 109.1 ± 71.1 | 80.4 ± 29.9 | 41.4 ± 8.8 |
| Female | 161.4 ± 48.3 | 112.9 ± 44.1 | 104.1 ± 36.0 | 55.4 ± 25.5 |
| Total | 152.7 ± 44.2 | 109.7 ± 57.4 | 92.3 ± 34.4 | 48.5 ± 20.3 |
Abbreviations: HDL, high density lipoprotein; LDL, low density lipoprotein.
aValues are expressed as mean ± SD.
No correlation was observed between fasting glucose levels and lipid profile. However, there was a significant relationship between fasting glucose levels and creatinine (r = 0.3, P < 0.03), and BUN (r = 0.5, P < 0.001). In addition, a significantly negative correlation was observed between the two groups in terms of uric acid (r = -0.5, P < 0.001) in the diabetic group.
5. Discussion
The results showed that level of fetuin-A decreased significantly in patients compared to healthy subjects (627.2 ± 649.0 mg/dL vs. 789.8 ± 716.7 mg/dL). Similar to our study, it has been reported that fetuin-A level was lower in persons with T2DM (14). Horng‐Yih et al. showed serum fetuin-A concentrations in newly diagnosed type 2 diabetes (NDD) and impaired glucose tolerance (IGT) groups were higher than normal glucose tolerance (NGT) (341 ± 88, 335 ± 90, and 300 ± 75 μg/mL, respectively). These results suggested, serum fetuin-A levels were positively related with IGT and NDD in subjects without nonalcoholic fatty liver disease (NAFLD) (5). It was also found that plasma fetuin-A level was elevated in patients with T2DM (15). It has been reported that fetuin-A caused insulin resistance by inhibiting insulin receptor autophosphorylation (4). Insulin resistance is an important factor in development of T2DM complications. Besides, insulin resistance is represented to be the reason of dyslipidemia and hypertension complications (8). Nevertheless, the function of fetuin-A and its contribution in insulin resistance is unclear. According to several studies, contradictory results have been reported based on the role of fetuin-A in complications of diabetes (6). In the present study, there was not a significant correlation between fetuin-A with other biochemical and demographic parameters. No relationship was observed between fetuin-A level and such factors as triglyceride, cholesterol, HDL levels, and BMI in patients with T2DM. Similar results have shown that there was not a significant correlation between different parameters such as BMI, blood pressure, total cholesterol, triglyceride, and HDL with serum fetuin-A in the diabetic patients (P > 0.05) (16).
Our study population included patients with advanced diabetes in terms of duration and presence of different variations in biochemical factors and other complications from six months to more than two years. It has been reported that higher levels of fetuin-A was associated with the improvement of cardiovascular disease (CVD) in the general population. It is suggested that high fetuin-A levels can cause peripheral arterial disease (PAD) (17). On the other hand, lower fetuin-A levels were associated with higher CVD risk in individuals without diabetes. Even though, there was no significant correlation in patients with T2DM. However, there was a trend toward higher CVD risk with higher fetuin-A levels (18).
Adipsin level showed a decrease in diabetic patients compared to control group (13 ± 11.7 mg/dL vs.18.0 ± 13.4 mg/dL, P = 0.001). These results were confirmed by Legakis et al. who reported that the circulating adipsin levels decreased in patients with T2DM (74.30 ± 12.51 pg/mL vs. 117.1 ± 5.03 pg/mL, P < 0.0001) (19). It is also reported that adipsin is a protein of adipose cells, but paradoxically declines in many animal models of obesity and diabetes (9). It has been reported that T2DM patients with β cell failure are deficient in adipsin (12). In the present study, it was also observed that adipsin level was inversely related with fasting glucose levels (r = -0.18, P = 0.08). In addition, there was not a correlation between adipsin level and lipid profile including; triglyceride, cholesterol, LDL, HDL, and also biochemical factors such as uric acid, creatinine, and B.U.N in diabetes. Adipsin has an important role in β cell function. In an experimental study, it was shown that genetic lack of adipsin occured with glucose intolerance due to insulin deficiency. In addition, administration of adipsin to diabetic animals which were hyperglycemic increaseed insulin secretion (12). Adipsin increases differentiation of adipocytes. It has been reported that the adipsin-acylation stimulating protein (ASP) system affects the regulation of triglyceride metabolism in adipocytes. It has also been shown that this system increases the level of triglyceride synthesis in adipocytes (9). Moreover, adipsin can act as a lipostatic signal based on the reduced expression of the adipsin gene and circulating protein levels in obese animals that was not supported in clinical data (20).
T2DM patients may be risk stratified based on their adipsin level, even when other glycemic indices such as the hemoglobin A1c are well-controlled. Adipsin is an adipokine that improves β cell function in diabetes. This group may need earlier insulin therapies. On the other hand, there may be a group of patients with high levels of adipsin who may be protected from T2DM. It seems that they are metabolically healthy but are obese (21, 22). Higher Fetuin-A concentrations were associated with gain of visceral adipose tissue, a major component of the metabolic syndrome (23).The level of serum fetuin-A are significantly associated with insulin resistance in women with T2DM. It has been reported that there is a significant correlation between fetuin-A and homeostasis model assessment–insulin resistance (HOMA-IR) index in women but not in men (24).
Fetuin-A and adipsin decreased in T2DM as compared to controls. In addition, higher fetuin-A levels are markedly related to metabolic syndrome (MetS) and an atherogenic lipid profile. However, future studies are necessary to assess whether fetuin-A can predict coronary artery disease risk (25).
Adipose tissue acts not only as energy storage in the body, but also as an endocrine organ that secretes different hormones or adipokines, which circulate in distinct target receptors in many tissues. These adipokines seem to contribute in the pathogenesis of T2DM (19).The different metabolic profile of the adipokines such as fetuin-A and adipsin improve the possible role of the adipose tissue in the energy metabolism and glucose homeostasis in T2DM. Different factors may contribute to fetuin-A regulation in relationship with the pathophysiological consequence in T2DM.
In the present study, it was revealed that there is a trend toward lower fetuin-A and adipsin concentrations in the patients. It has been suggested that fetuin-A and adipsin levels could play a role in the development of type 2 diabetes complications. Besides, in comparison with published data the changes of fetuin and adipsin levels in patients with T2DM indicate a complex but not complete role of fetuin-A and adipsin in the pathogenesis of T2DM. On the other hand, there was a mild to moderate increase in cholesterol and triglyceride levels which require further studies. It seems, the result of fetuin-A and adipsin levels in diabetes depends on numerous vague factors. Although this study is cross-sectional and can not run fundamental consequences, it might help us understand the role of following adipokines in the pathophysiology of diabetic patients as compared with control and be the call for further studies designed to clarify the exact mechanism.
There are several limitations to this study, including the cross-sectional nature of the study, limited sample size, and lack of information on the genetic and hormonal characteristics of the subjects. Therefore, it is difficult to present a fundamental relationship according to this cross-sectional design. Nevertheless, further studies are needed to understand factors affecting these adipokine levels during disease.